Preparation and amplification culture method of human multipotential stem cell sourced human retinal pigment epithelium cells
A technology of human pluripotent stem cells and retinal pigment, applied in the field of stem cells, can solve the problems of lack of RPE cells restricting development, and achieve the effects of low cost, high yield and simple technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Expansion and culture of human pluripotent stem cells
[0041] Three human pluripotent stem cell (hPSC) lines were used for the study. BC1-GFP-hiPSC and BC1-hiPSC were gifts from friends, and the other hiPSC was purchased from American Life Technology Company ( Episomal hiPSC Line, A18945). All cells were seeded on 6-well culture plates coated with extracellular matrix MatriGel (Corning, 354277), and expanded and cultured with mTeSR1. When the cell confluency reaches 80%-90%, it is digested with 0.5mM EDTA (Life, 15575-038), and subcultured at 1:8 to 1:12. Observed under an inverted microscope, the cells were in sheet form, growing like colonies, and the cells in the colonies were tightly arranged with unclear borders ( figure 1 ), thereby expanding human pluripotent stem cells (hPSCs).
Embodiment 2
[0042] Example 2: Induction of differentiation of hPSCs into retinal cells including pigment epithelial cells
[0043] Referring to the reported method (reference: Xiufeng Zhong, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from humaniPSCs. Nat Commun. 2014 Jun 10; 5:4047), hPSCs were induced to differentiate into retinal cells including RPE cells. The day when hPSCs start to differentiate (that is, the hPSC expansion medium is replaced with differentiation medium or embryoid bodies are prepared) is set as the "0" day of differentiation.
Embodiment 3
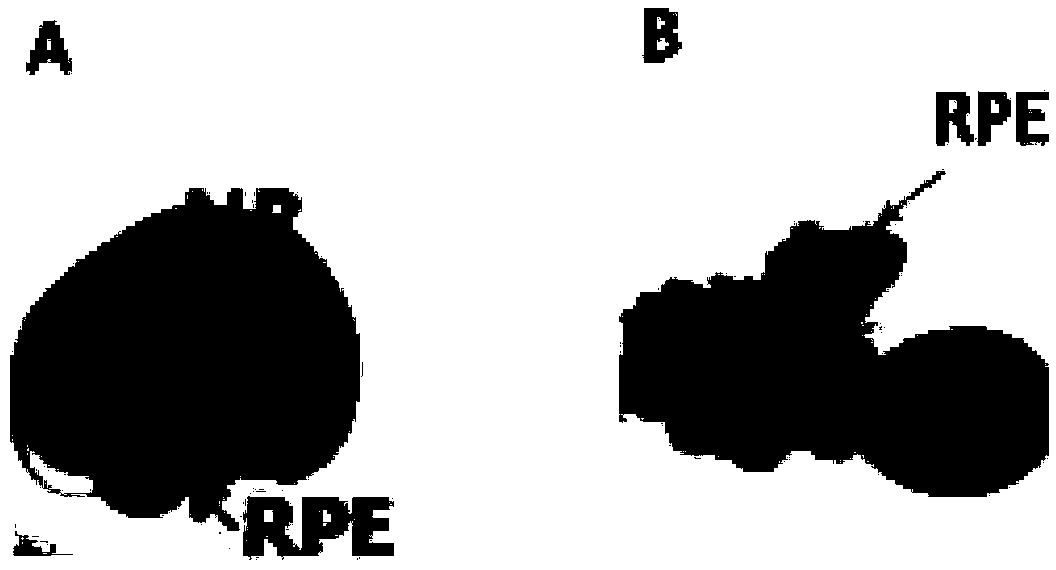
[0044] Example 3: Obtaining and culturing of hPSC-derived 3D-RPE spheres
[0045] The retinal cells at the 4th week of hPSC-induced differentiation included RPE cells, and the neural retina (NR) and RPE with slight bulge, ring, and strong refraction could be recognized under the microscope. RPE can grow around NR, or it can grow individually or in sheets ( figure 2 ). Use a self-made tungsten needle or a 1mL syringe to stir up NR and its nearby RPE, and transfer it to a low-adsorption petri dish for suspension culture. The culture medium is RPE cell culture medium. Its formula is: every 100mL cell culture medium contains 10mL fetal bovine serum (Gibco, 10099-141), 2mL 50×B27 nerve cell growth supplement (Gibco, 12587-010), 1mL 100× penicillin-streptomycin mixed solution (Gibco, 15240), 1 mL of 100× non-essential amino acids (Gibco, 11140-050), 1 mL of 100× glutamine (Gibco, 35050-061) and 0.1 mL of 1000× taurine (Sigma, T-0652), the balance being DMEM / F12 (3:1) medium, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com