Compositions and methods for donor selection and prognosis of acute graft-versus-host disease
A donor, prognostic technology, applied in the fields of biochemical equipment and methods, microbial determination/examination, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Sample collection, RNA extraction, reverse transcription, real-time PCR and sequencing of PCR products
[0110] The study was performed using a cohort consisting of 192 human subjects undergoing allogeneic HCT and 114 corresponding donors from multiple centers. Plasma samples were collected from Duke University Medical Center, Dana-Farber Cancer Institute, and the Blood and Marrow Transplant Clinical Trials Network. The miRNA profiling set consisted of 19 HCT patients at Duke University Medical Center who had developed aGVHD and 23 HCT patients who had never developed aGVHD (non-GVHD). Another cohort of 15 non-GVHD and 15 aGVHD pre-onset aGVHD samples from Dana-Farber Cancer Institute was used to perform independent open array assays to validate candidate miRNAs identified from Duke samples. The miR-142-3p identification set included 6 aGVHD and 6 non-GVHD patients, and a corresponding donor for each recipient from the Dana-Farber Cancer Institute. The miR-142-3p vali...
Embodiment 2
[0119] Profiling of candidate miRNAs using a high-throughput platform
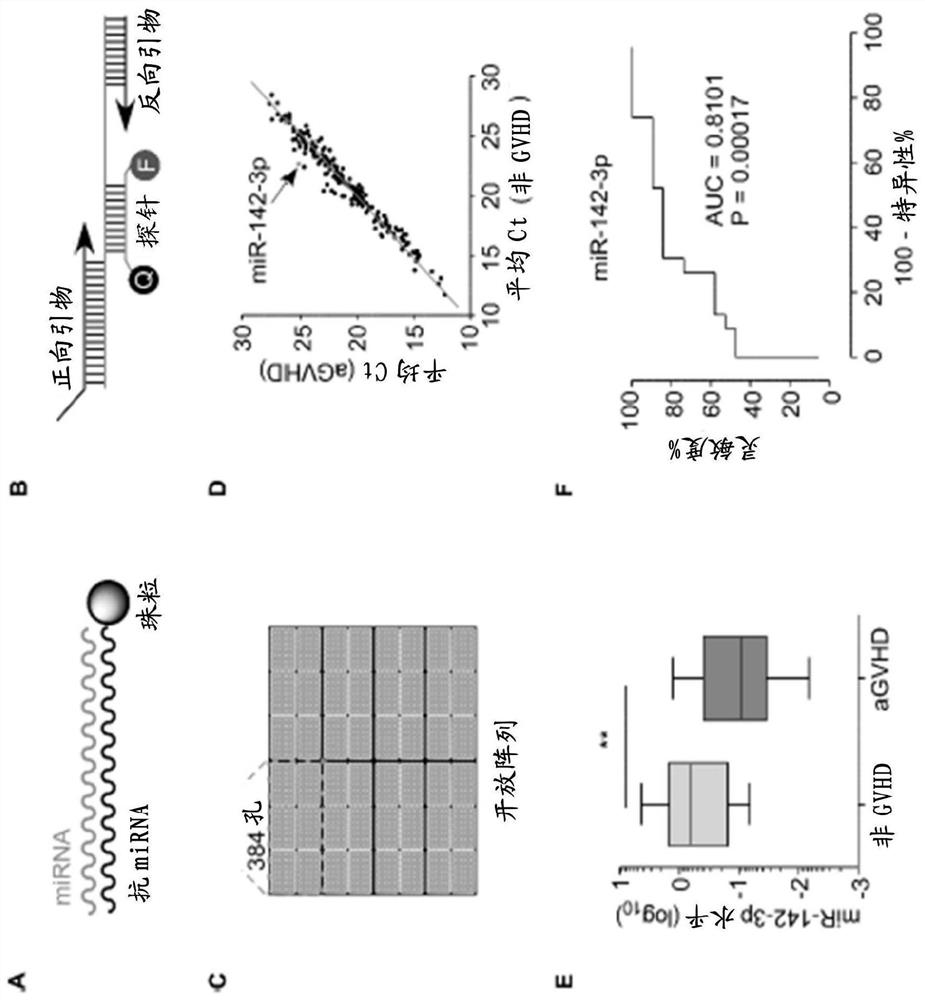
[0120] The microRNA sequence-specific anti-miRNA bead capture method was used to purify microRNA from plasma ( figure 1 A). RT-PCR inhibitors and degraded RNA fragments commonly found in blood-related samples are washed out from the final eluate. In addition, probe-based real-time PCR methods ( figure 1 B) and open array PCR chip ( figure 1 C) for improved specificity and throughput. The platform was validated by sequencing five random microRNA PCR products from plasma microRNAs from healthy donors. Sequencing results showed that the platform could efficiently and specifically detect microRNAs from plasma samples (92%<specificity≤100%). MicroRNA profiling was performed on the platform using plasma from 19 patients who had developed GVHD and 23 timepoint-matched non-GVHD subjects.
[0121] Most plasma microRNAs showed similar expression patterns between disease and non-disease ( figure 1 D). General...
Embodiment 3
[0124] Donor and recipient plasma miR-142-3p ratios are associated with aGVHD development
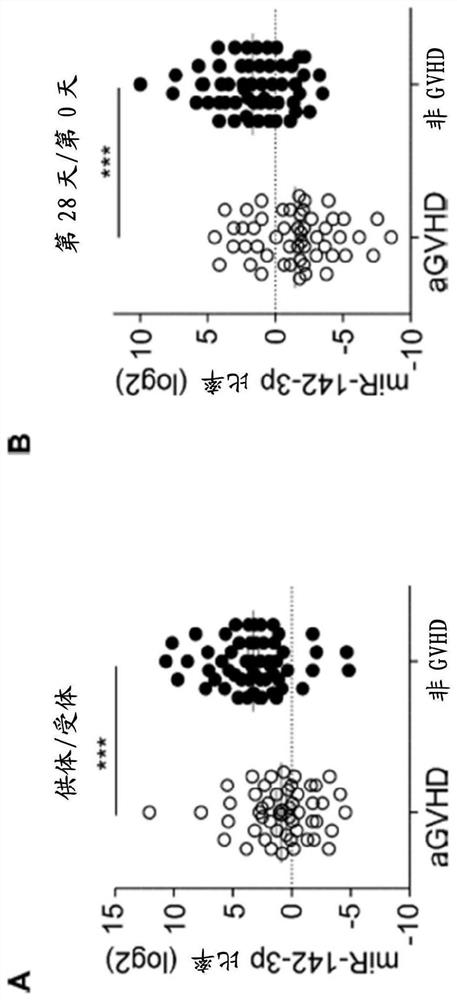
[0125] The expression level of miR-142-3p in plasma from aGVHD patients was determined to be lower ( figure 1 D, 1E). The ratio of plasma miR-142-3p between donors and recipients was on average 4-fold lower in the aGVHD group compared with those from the non-GVHD group. The expression level of miR-142-3p was also determined using plasma samples obtained on days 0 and 28 after BMT. In both cases, a low ratio of miR-142-3p levels in plasma before BMT was associated with aGVHD ( figure 2 A). Furthermore, the miR-142-3p ratio between day 0 and day 28 after BMT showed a similar pattern between the aGVHD group and the non-GVHD group ( figure 2 B). These data suggest that low miR-142-3p levels in donor plasma are a risk factor for the development of aGVHD after donor selection and BMT. Furthermore, plasma miR-142-3p levels of recipients after BMT can be used to monitor the development ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com