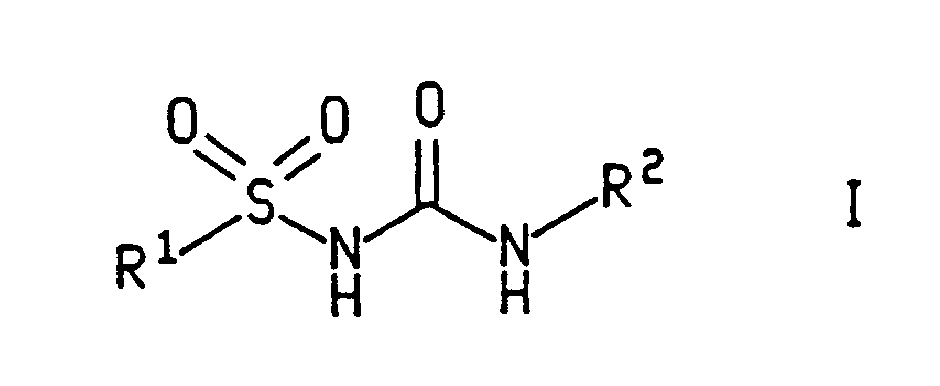
Sulfonyl urea derivatives and their use in control of interleukin-1 activity
A compound, C1-C6 technology, applied in the direction of sulfonylurea active ingredients, organic compound preparation, organic active ingredients, etc., can solve joint pain, diffuse myalgia, anorexia and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0150] The following reaction schemes illustrate the preparation of the present invention. Unless otherwise stated, n, A, B, D, E and G in these reaction schemes and in the discussion that follows are as defined above.
[0151] Preparation A
[0152] Preparation B
[0153] Preparation C
[0154] Preparation D
[0155] plan 1 In Reaction 1 of Preparation A, the compound of formula XII is mixed with triphosgene in such as triethylamine, diisopropylethylamine or 1,8-diazabicyclo[5,4,0]undec-7-ene, etc. Base, and in the presence of an aprotic solvent such as tetrahydrofuran, benzene or dichloromethane, the compound of formula XII is converted into the corresponding isocyanate compound of formula XI. The mixture is stirred and heated to reflux for about 1 to about 3 hours, preferably about 2 hours.
[0156]In reaction 1 of Preparation B, the compound of formula XIV is converted to the corresponding sulfonamide compound of formula XIII by the following method: at a te...
Embodiment 1
[0333] Example 11-(4-chloro-2,6-diisopropyl-phenyl)-3-[3-(1-hydroxyl-1-methylethyl)-benzenesulfonyl]-
[0334] Urea
[0335] To a stirred solution of 2-(3-aminosulfonylphenyl)-propan-2-ol (26.5 g) in tetrahydrofuran was added sodium hydride (5.2 g of a 60% dispersion in mineral oil) in portions. Once hydrogen evolution had ceased, 4-chloro-2,6-diisopropylphenylisocyanate (30.8 g) was added in one portion and the resulting mixture was heated at reflux for 12 hours. The mixture was then cooled to room temperature and concentrated in vacuo. The resulting foam was dissolved in water, made basic with 1N sodium hydroxide, and extracted with two 1:1 portions of ether / hexane. The aqueous layer was acidified with 1N hydrochloric acid and the resulting white solid was filtered, washed with water and dried. This gave 50 g of a white solid, which was recrystallized from wet ethyl acetate / hexane to give the title compound, mp 160.5-162.0°C.
Embodiment 2
[0337] Example 21-(4-chloro-2,6-diisopropyl-phenyl)-3-[3-(1-hydroxycyclopentyl)-benzenesulfonyl]-urea
[0338] 3-1-Hydroxycyclopentyl-benzenesulfonamide; 4-chloro-2,6-diisopropyl-phenylisocyanate. mp: 155°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com