Anti-human vista antibodies and use thereof
An antibody and antibody fragment technology, applied in the field of anti-human VISTA antibody and antibody fragment, anti-human VISTA antibody and antibody fragment, can solve the problem that anti-human VISTA antibody or antibody fragment has not been identified yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
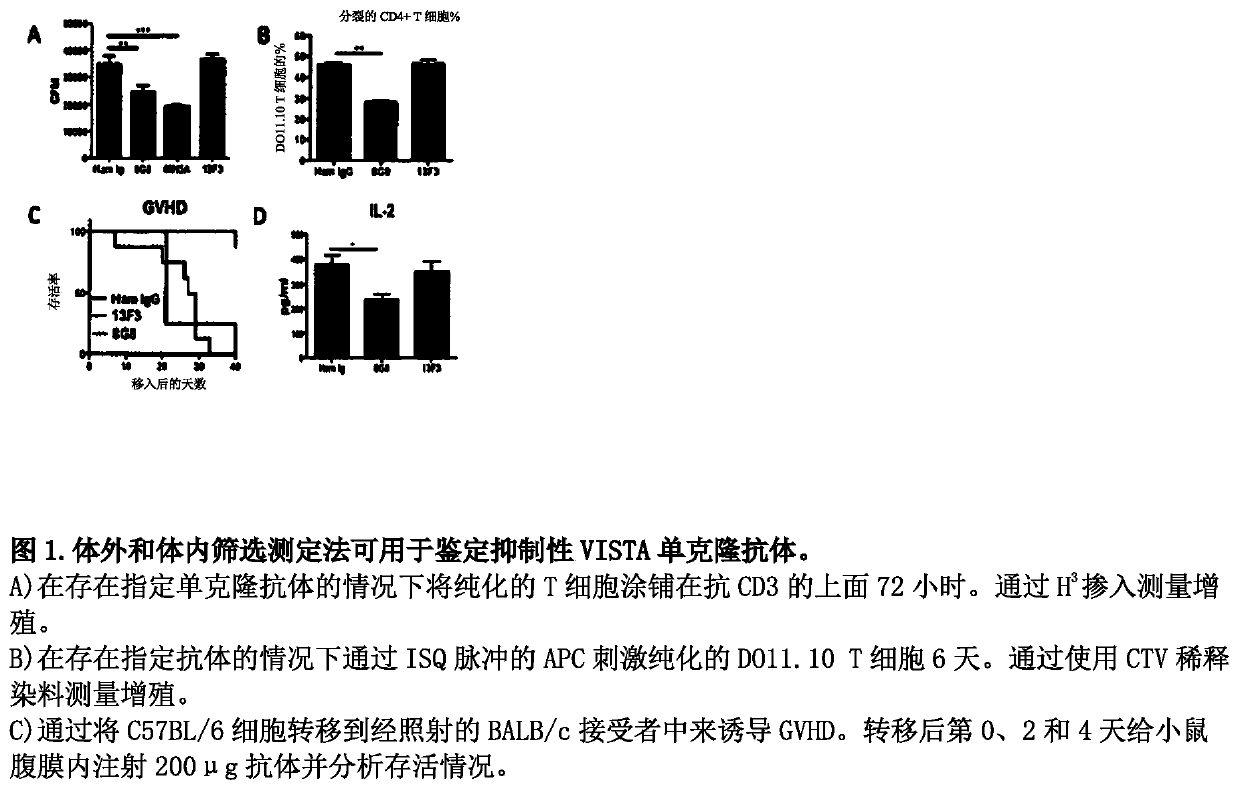
[0592] Example 1: Assay to screen for immunosuppressive anti-mouse VISTA antibodies
[0593] The inventors developed various assays for screening putative agonistic anti-mouse VISTA antibodies. like figure 1 In vitro and in vivo screening assays were used to identify immunosuppressive anti-VISTA monoclonal antibodies as shown in . exist figure 1 In experiments in A, purified T cells were plated on anti-CD3 in the presence of the indicated monoclonal antibodies for 72 hours. Proliferation was measured by H3 incorporation. exist figure 1 In the experiments of B, purified DO11.10T cells were stimulated by ISQ-pulsed APCs in the presence of the indicated antibodies for 6 days. Proliferation was measured by diluting the dye with CTV. exist figure 1 In the experiments in C, GVHD was induced by transferring C57BL / 6 cells into irradiated BALB / c recipients. Mice were injected intraperitoneally with 200 μg of antibody on days 0, 2, and 4 post-transfer and analyzed for survival...
Embodiment 2
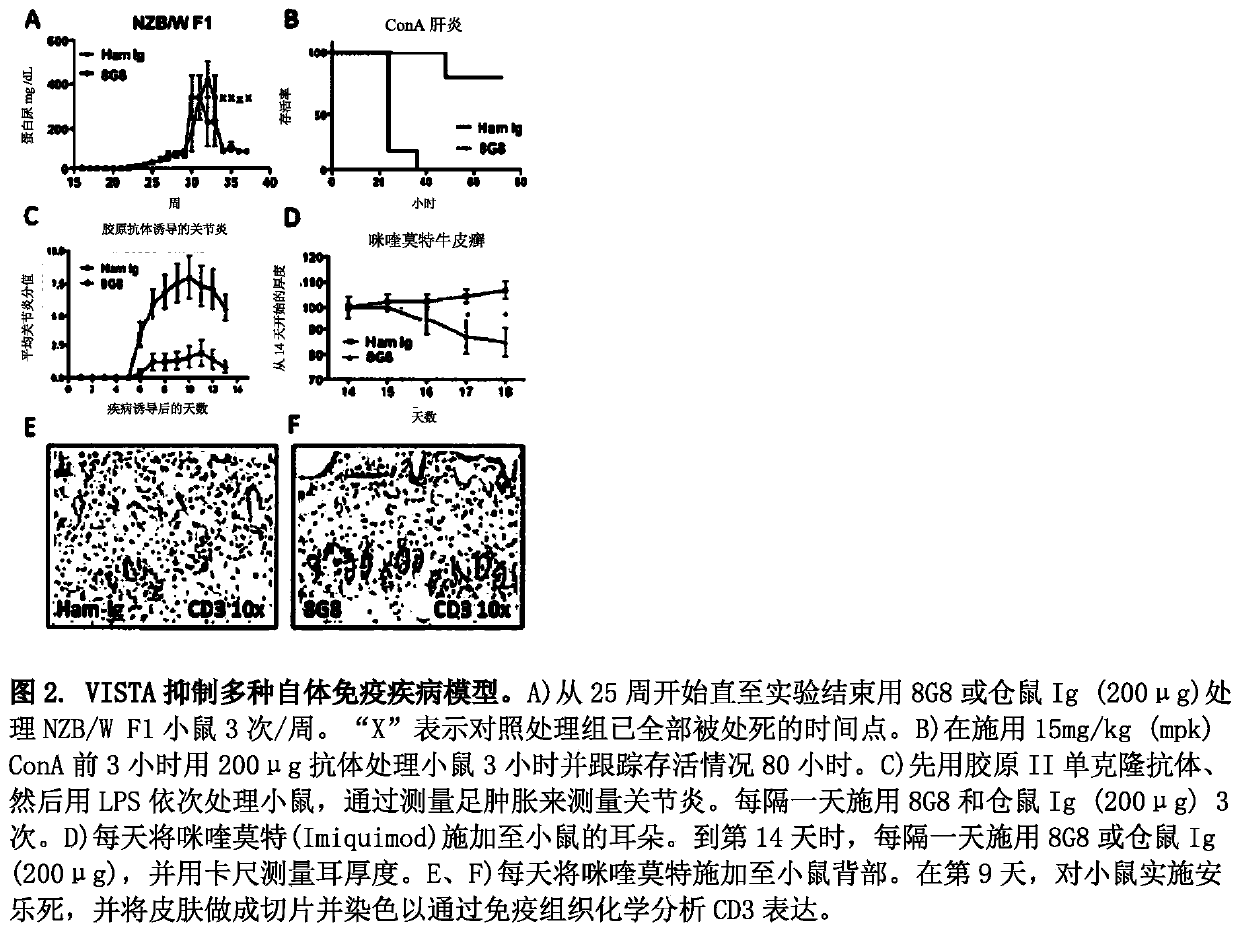
[0598] Example 2: Identification of anti-VISTA antibodies that suppress autoimmunity in different autoimmune disease models
[0599] exist figure 2 In the experiments in A-F, the effects of different anti-mouse VISTA antibodies were again compared in different disease models. exist figure 2 In the experiment in A, NZB / WF1 mice were treated with Ab1 or hamster Ig (200 μg) 3 times / week from week 25 until the end of the experiment. "X" indicates the time point at which the control treatment group was all sacrificed. exist figure 2 In the experiment in B, mice were treated with 200 μg of antibody 3 hours before administration of 15 mg / kg (mpk) ConA for 3 hours and followed for survival for 80 hours. exist figure 2 In the experiments in C, mice were sequentially treated with Collagen II mAb followed by LPS, and arthritis was measured by measuring foot swelling. In these experiments, Ab1 and hamster Ig (200 μg) were administered 3 times every other day. exist figure 2...
Embodiment 3
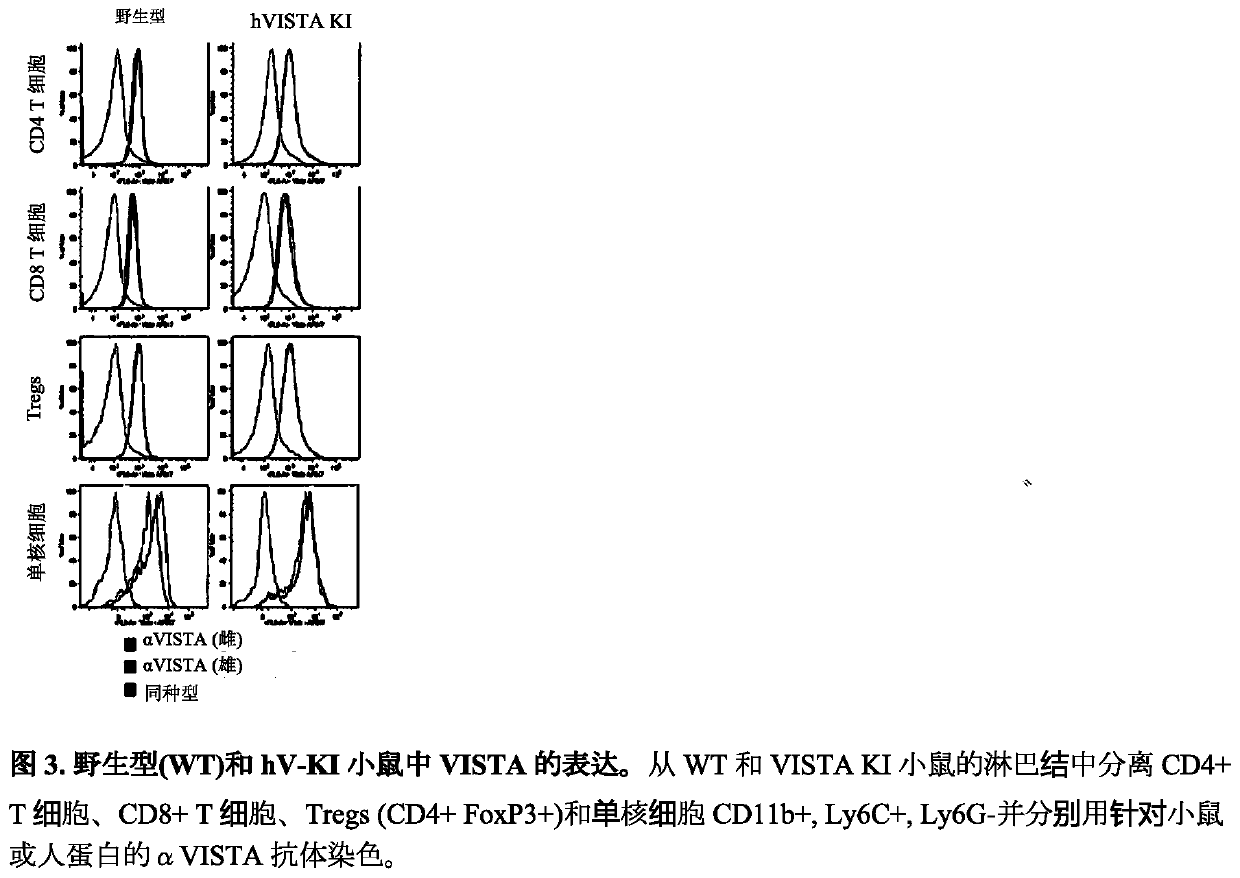
[0605] Example 3: Development of human VISTA knock-in mice for screening of agonistic anti-human VISTA antibodies
[0606] The previous examples relate to the isolation and characterization of agonistic anti-mouse VISTA antibodies. To date, agonistic anti-human VISTA antibodies have never been reported in the literature. Although the present assignee and other groups have identified a very large number of antagonistic anti-human VISTA antibodies. Therefore, prior to the present invention, it was uncertain whether agonistic anti-human VISTA antibodies would be identified.
[0607] Such antibodies would be very beneficial as there are currently no approved human therapeutics that exploit the natural function of the NCR to suppress immune responses. Although Orencia (CTLA4-Ig) is potent, it works only by blocking the CD28-B7 interaction and pathway, and not by stimulating the down-regulated pathway. The involvement of this pathway may prove to be a revolution in the manageme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com