Kit for early warning rejection reaction after kidney transplant and use method of kit
A technology of rejection reaction and kit, which is applied in the field of transplant kidney rejection early warning kit, which can solve the problems of complex, difficult and difficult diagnosis of transplant rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 Screening of Significant Difference Markers in the Early Diagnosis Kit of Transplanted Kidney Rejection
[0106] 1. Collect serum of kidney transplant recipients at multiple time points after transplantation, and analyze and group clinical cases.
[0107] 1.1 Select kidney transplant recipients with preoperative basic diseases or few complications, and no serious complications during the operation, and select kidney transplant recipients at 1 day, 7 days, 14 days, 21 days, 28 days, and 2 months after transplantation. 1. Collect 2ml of whole blood from 3 months to 12 months, let it stand at room temperature for 1 hour, centrifuge at 1000rpm for 10 minutes to collect the supernatant, and store it in a -80°C refrigerator for later use. In addition, the serum of the patients in the acute rejection group after the diagnosis of acute rejection was collected at any time.
[0108]1.2 According to the comprehensive analysis and case screening of serum creatinine, urea...
Embodiment 2
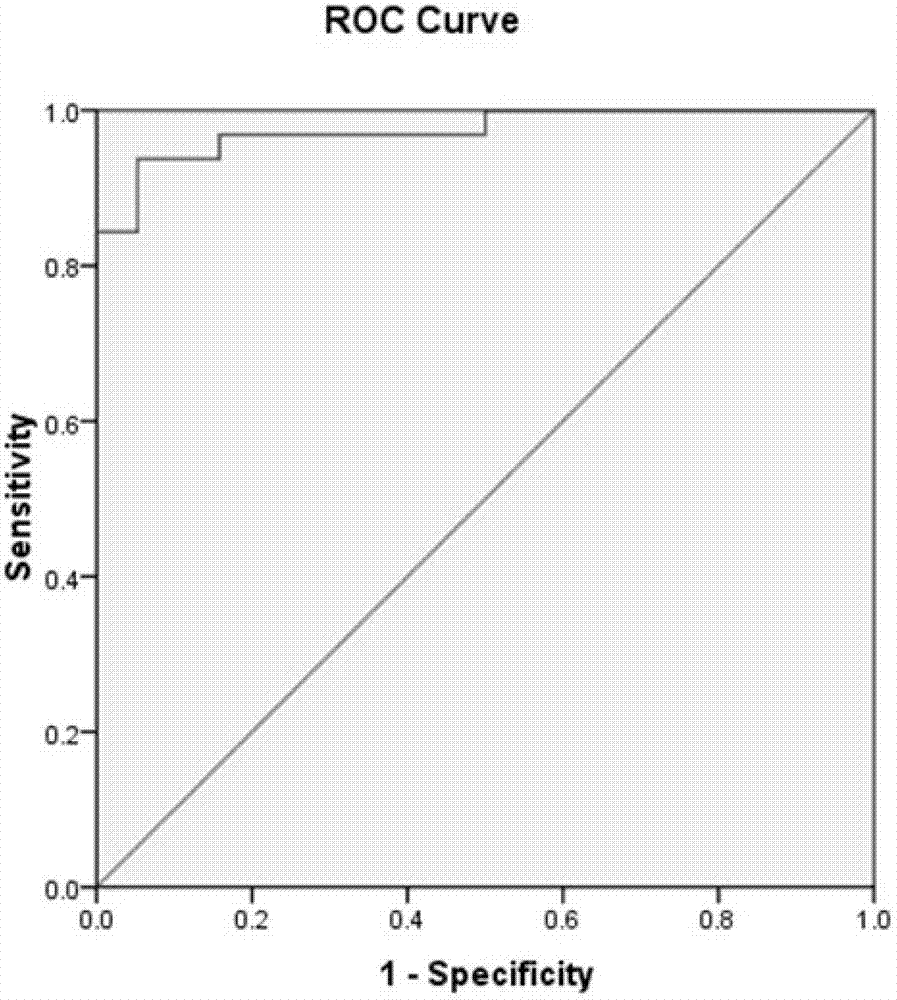
[0124] The 35 markers with significant differences obtained in Example 1 were analyzed, and the markers with significant differences in expression levels were analyzed by logistic regression to obtain 4 factors: SCF, sEGFR, sIL2Ralpha and Eotaxin. The early warning and diagnosis system of acute rejection, the statistical analysis shows that the predicted positive rate is 91.4. The area under the ROC diagnostic curve is 97.5%, see appendix figure 1 .
[0125] Wherein, the concrete composition of kit is:
[0126] (1) 96-well filter plate and two sheets of sealing film; purchased from Millipore, Cat. No. MX-PLATE;
[0127] (2) Standards of protein antibodies in the kit; purchased from Millipore, Cat. No. MXH8060, MXH8062, MXH8063, LHSP-8063 and HSCR-8032;
[0128] (3) Quality control control; purchased from Millipore Company, the product numbers are: MXH6060, MXH6062, MXH6063, LHSP-6063 and HSCR-6032;
[0129] (4) Serum matrix; purchased from Millipore Company, the article nu...
Embodiment 3
[0136] Example 3: Composition of the kit for early diagnosis of acute rejection of transplanted kidney
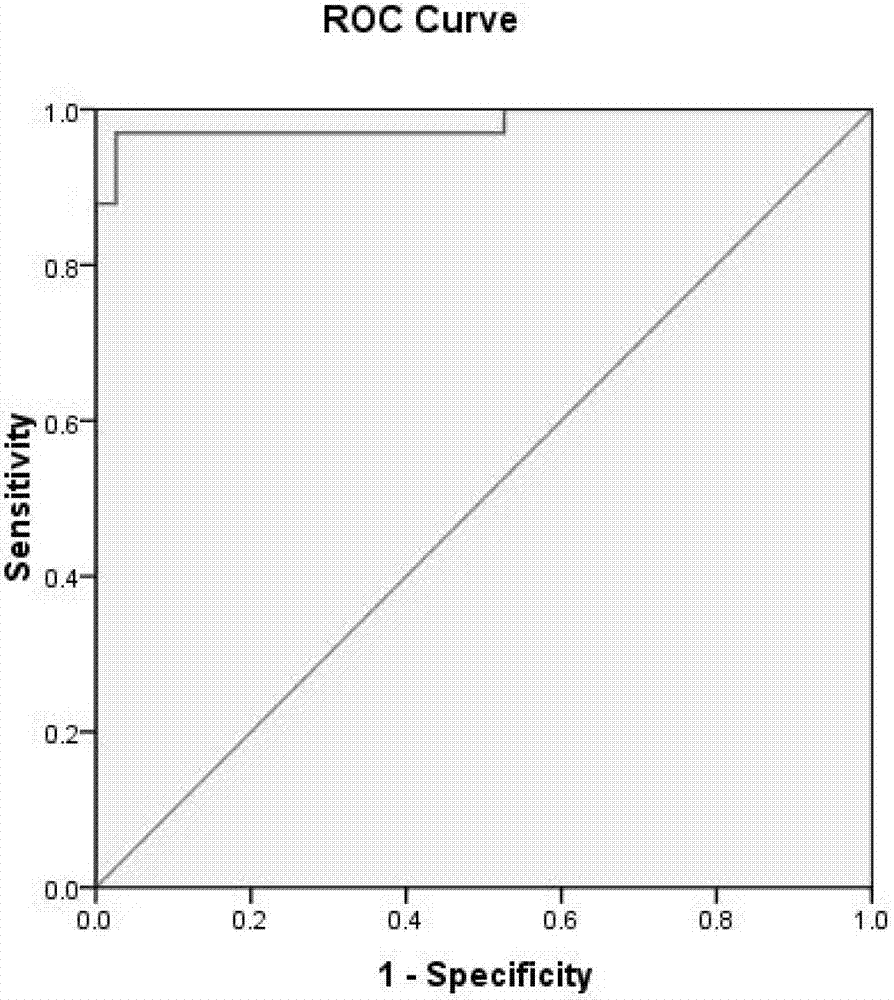
[0137] The 35 markers with significant differences obtained in Example 1 were analyzed, and 14 factors were obtained after logistic regression analysis on the markers with significant differences in expression levels: sTNFR2, Flt3Ligand, Fractalkine, IL1ra, IL2, MDC , MIP1alpha, SDF1alphabeta, TARC, TRAIL, SCF, CCL20, MIP3alpha and XCL1Lymphotactin; statistical analysis shows that the early diagnosis system predicts a positive rate of 98.6%, and the area under the ROC diagnostic curve is 99.8%. figure 2 .
[0138] Wherein, the concrete composition of kit is:
[0139] (1) 96-well filter plate and two sheets of sealing film; purchased from Millipore, Cat. No. MX-PLATE;
[0140] (2) Standards of protein antibodies in the kit; purchased from Millipore, Cat. No. MXH8060, MXH8062, MXH8063, LHSP-8063 and HSCR-8032;
[0141] (3) Quality control control; purchased from Millipore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com