Steroid saponin pharmaceutical composition and its preparation method and uses
A technology of steroidal saponin and composition, applied in the field of steroidal saponin pharmaceutical composition, can solve the problems of environmental pollution, a large number of organic solvents, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
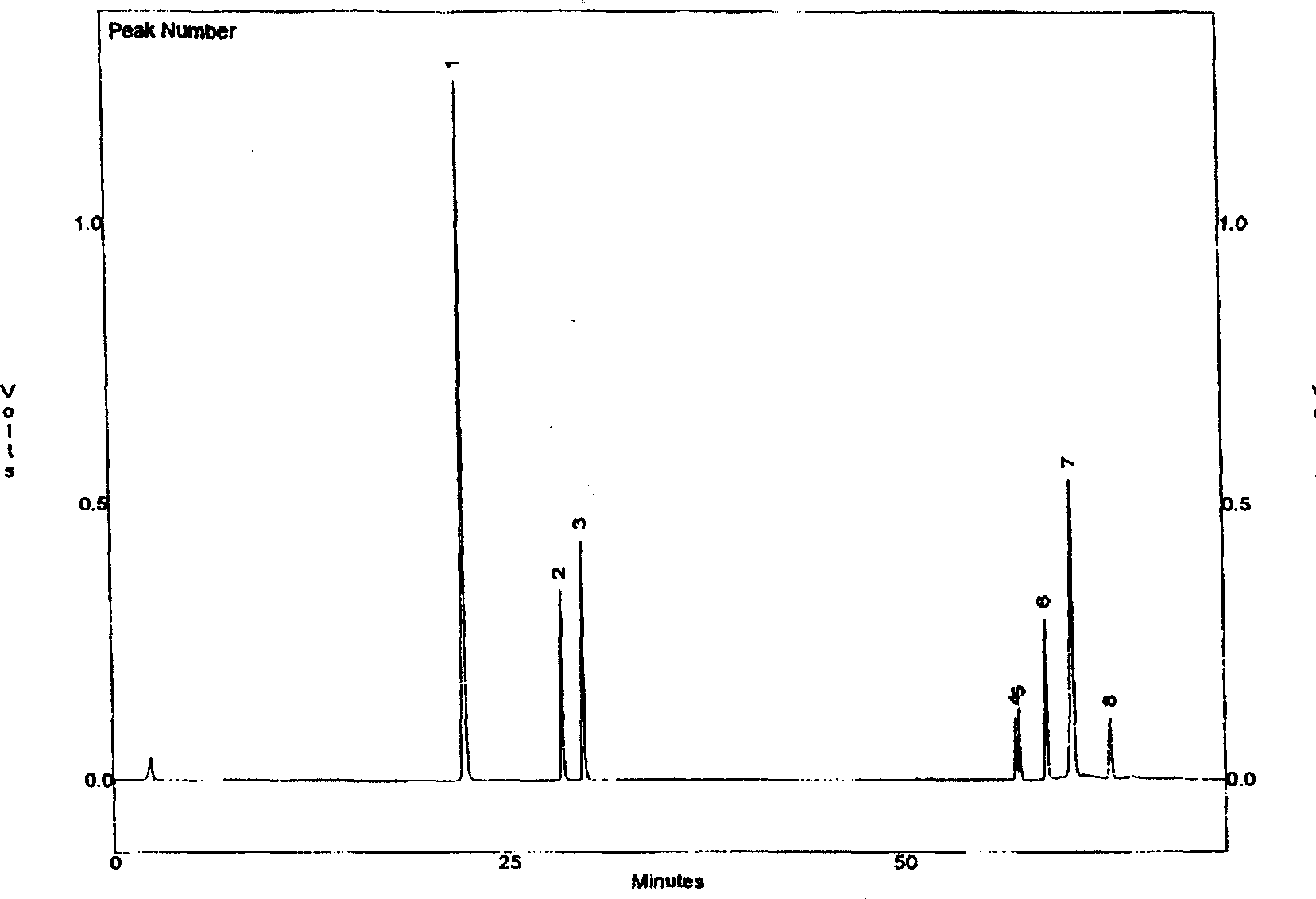
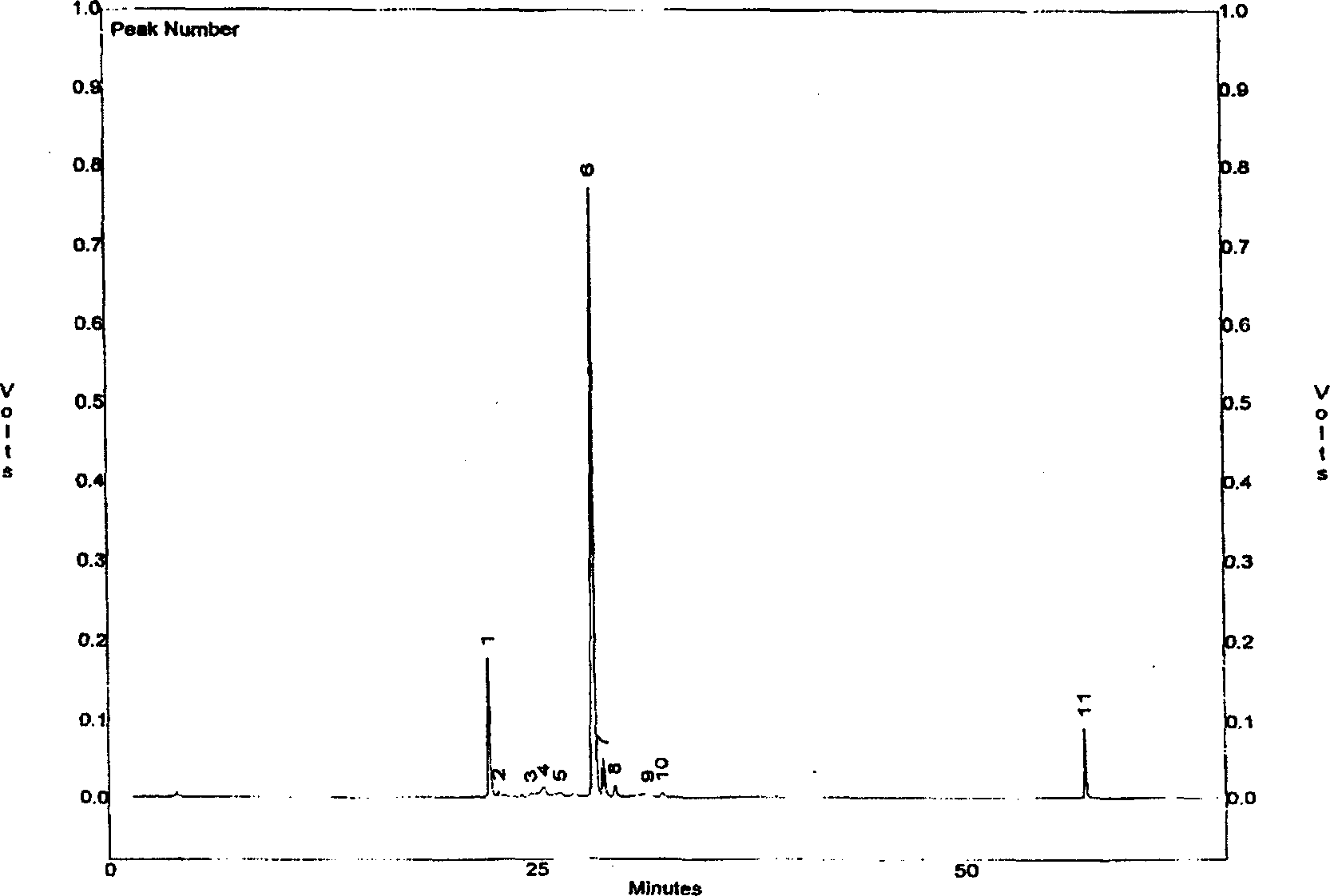
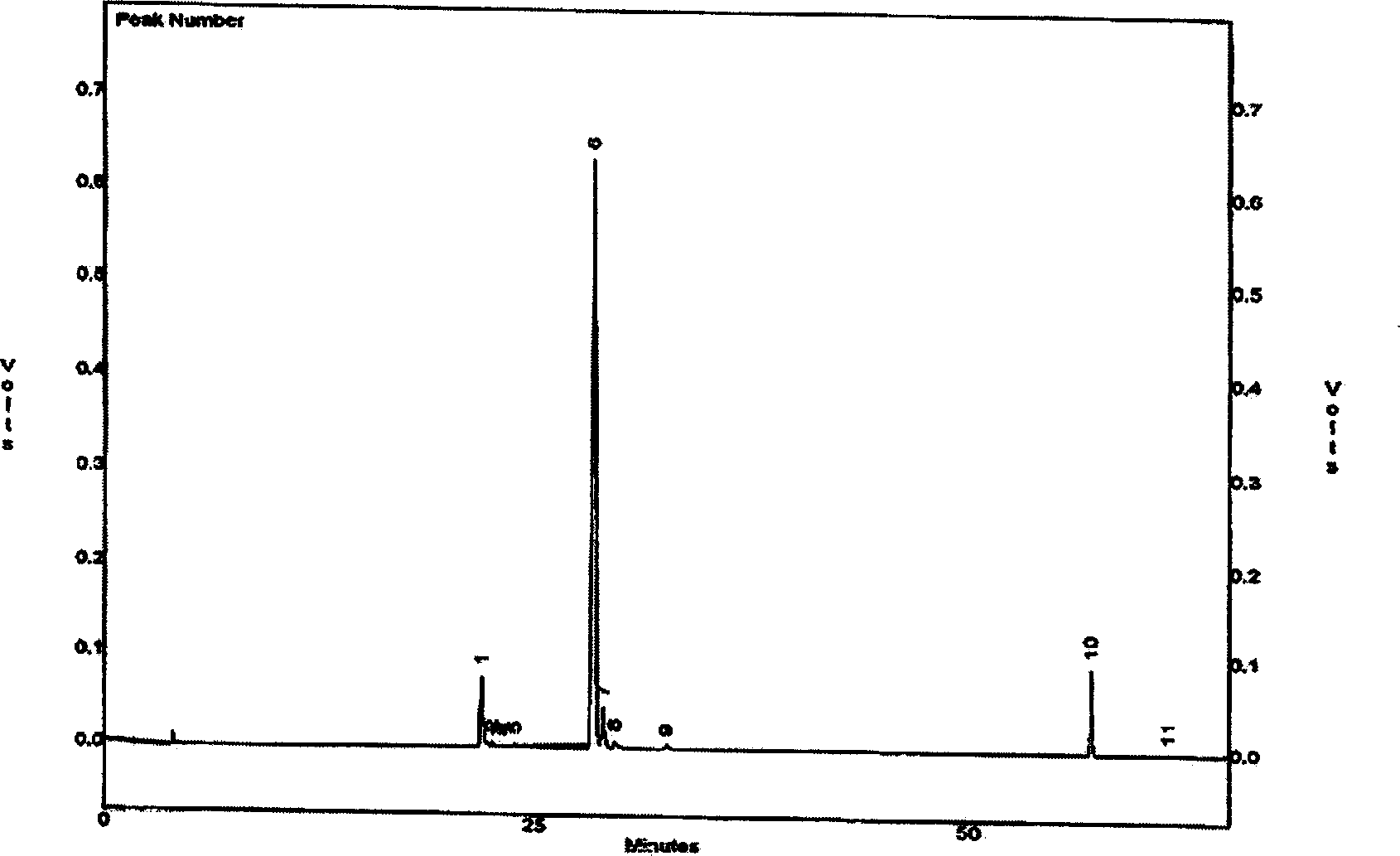
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 pharmaceutical composition of the present invention:
[0043] 100kg of dried rhizomes of the traditional Chinese medicine Pangosia chinensis, pulverized, refluxed with 95% ethanol (1200L × 3), 3 hours for the first time, 2 hours for the second and third times, filtered, combined extracts; recovered ethanol, added water to 2g / ml, Refrigerate for 24 hours, centrifuge; supernatant through HPD 300 The adsorption resin column is first washed with water until the effluent is colorless, and then eluted with 70% ethanol; the eluted part of 70% ethanol is received, the ethanol is recovered and concentrated to a thick extract, and then vacuum-dried to obtain the total saponins of pangolin steroid. The yield was 2.32%.
Embodiment 2
[0044] The preparation of embodiment 2 pharmaceutical compositions of the present invention
[0045] 100kg of dried rhizome of Chinese yam, sliced, decocted with 12 times (V / W) water 4 times, 3 hours for the first time, 2 hours for the second, third, and fourth times, filtered, and combined extracts; Centrifuge at room temperature; the supernatant is passed through a macroporous adsorption resin column [HPD 100 / LD 140 =7:3 (W / W)], wash with water until the effluent is colorless, then elute with 10% ethanol, and finally elute with 75% ethanol; receive the eluted part of 75% ethanol, and recover ethanol until it is free of alcohol taste, and then spray-dried to obtain the total steroidal saponins of Chinese yam with a yield of 1.15%.
Embodiment 3
[0046] The preparation of embodiment 3 pharmaceutical composition of the present invention
[0047] 400kg of dried rhizomes of fresh pangosaurus, sliced, decocted with 8 times (V / W) water for 3 times, the first time for 3 hours, the second and third times for 2 hours each, filtered, combined extracts; put to room temperature, centrifuged; The supernatant was passed through a macroporous adsorption resin column [HPD 100 / D 101 = 6:4 (W / W)], first wash fully with water, then elute with 10% ethanol, and finally elute with 80% ethanol; receive the eluted part of 80% ethanol, recover the ethanol until it has no alcohol smell, After spray drying, the total pangolin steroid saponins were obtained with a yield of 0.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com