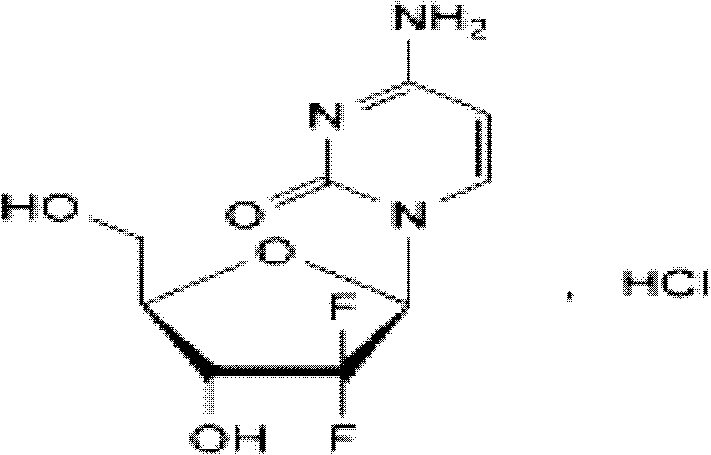
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
A technology of gemcitabine hydrochloride and freeze-dried powder injection, applied in the field of pharmaceutical preparations, can solve the problems of unsuitable popularization, high production cost, long freeze-drying cycle and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
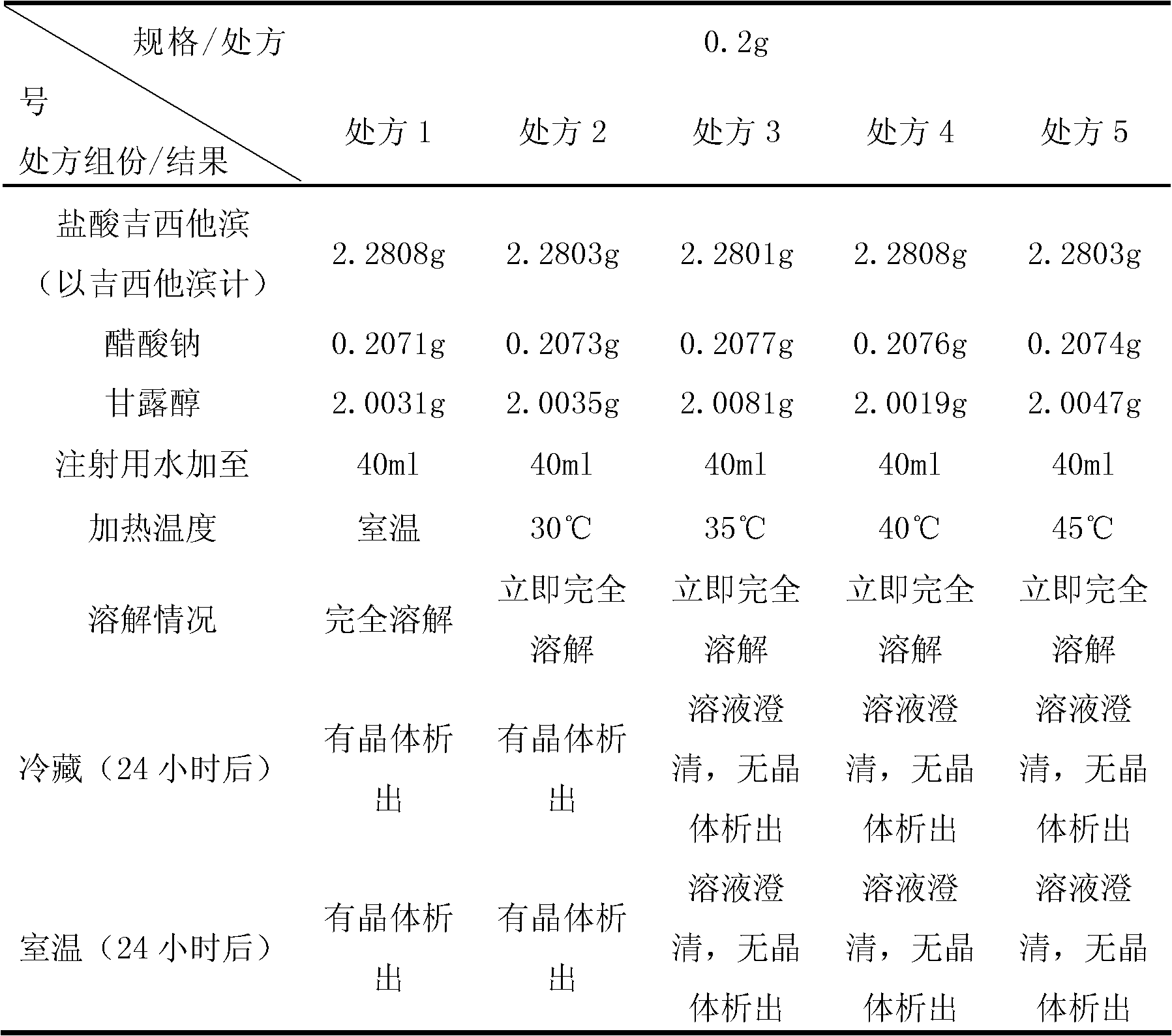
[0075] (1) Prescription:
[0076]
[0077] (2) Preparation process:
[0078] Weigh prescription amounts of gemcitabine hydrochloride, sodium acetate, lactose and mannitol. First add 85% of the total amount of water for injection into the liquid mixing tank, put in the materials, stir to dissolve completely; control the temperature of the solution in the liquid mixing tank at 38°C, adjust the pH to 2.7-3.3, and replenish the water for injection to the full amount , mix evenly, add medicinal activated carbon, stir and absorb, decarbonize, after the intermediate is qualified, the liquid is sterilized and filtered at 0.22 μm, and then the liquid is moved to the 100-level laminar flow hood of the filling machine for a second time at 0.22 μm Terminally sterilized and filtered into the liquid medicine barrel, filled, half-tamped, and freeze-dried.
[0079] Pre-freezing stage: reduce the shelf temperature to -50°C at a constant rate within 3 hours, and keep warm for 5 hours after...
Embodiment 2
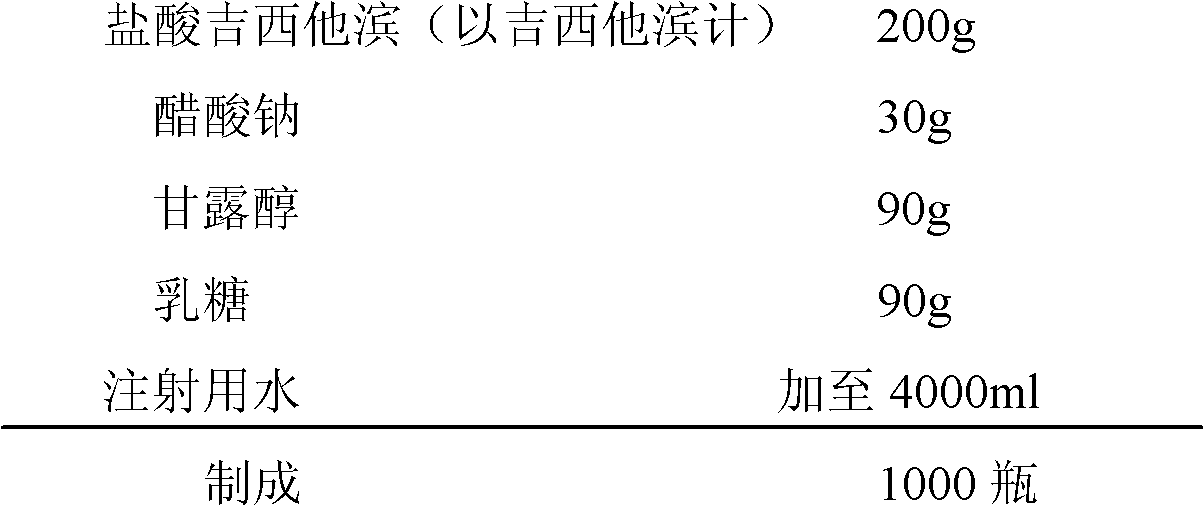
[0083] (1) Prescription:
[0084]
[0085] (2) Preparation process:
[0086] Weigh prescription amounts of gemcitabine hydrochloride, sodium acetate, lactose and mannitol. First add 85% of the total amount of water for injection into the liquid mixing tank, put in the materials, stir to dissolve completely; control the temperature of the solution in the liquid mixing tank at 45°C, adjust the pH to 2.7-3.3, and replenish the water for injection to the full amount , mix evenly, add medicinal activated carbon, stir and absorb, decarbonize, after the intermediate is qualified, the liquid is sterilized and filtered at 0.22 μm, and then the liquid is moved to the 100-level laminar flow hood of the filling machine for a second time at 0.22 μm Terminally sterilized and filtered into the liquid medicine barrel, filled, half-tamped, and freeze-dried.
[0087] Pre-freezing stage: reduce the shelf temperature to -48°C within 3 hours, and keep warm for 4 hours after the product temperat...
Embodiment 3
[0091] (1) Prescription:
[0092]
[0093]
[0094] (2) Preparation process:
[0095] Weigh prescription amounts of gemcitabine hydrochloride, sodium acetate, lactose and mannitol. First add 85% of the total amount of water for injection into the liquid mixing tank, put in the materials, stir to dissolve completely; control the temperature of the solution in the liquid mixing tank at 30°C, adjust the pH to 2.7-3.3, and replenish the water for injection to the full amount , mix evenly, add medicinal activated carbon, stir and absorb, decarbonize, after the intermediate is qualified, the liquid is sterilized and filtered at 0.22 μm, and then the liquid is moved to the 100-level laminar flow hood of the filling machine for a second time at 0.22 μm Terminally sterilized and filtered into the liquid medicine barrel, filled, half-tamped, and freeze-dried.
[0096] Pre-freezing stage: reduce the shelf temperature to -50°C within 3 hours, and keep warm for 3 hours after the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com