TS-1 granules and preparation method thereof
A technology of granules and Gio, applied in the field of medicine, can solve problems such as the stability and water solubility of drugs are not improved, the water solubility of drugs is not improved, and the dissolution rate of Sigiol is affected, so as to improve the bioavailability and improve the Water solubility, the effect of maintaining drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
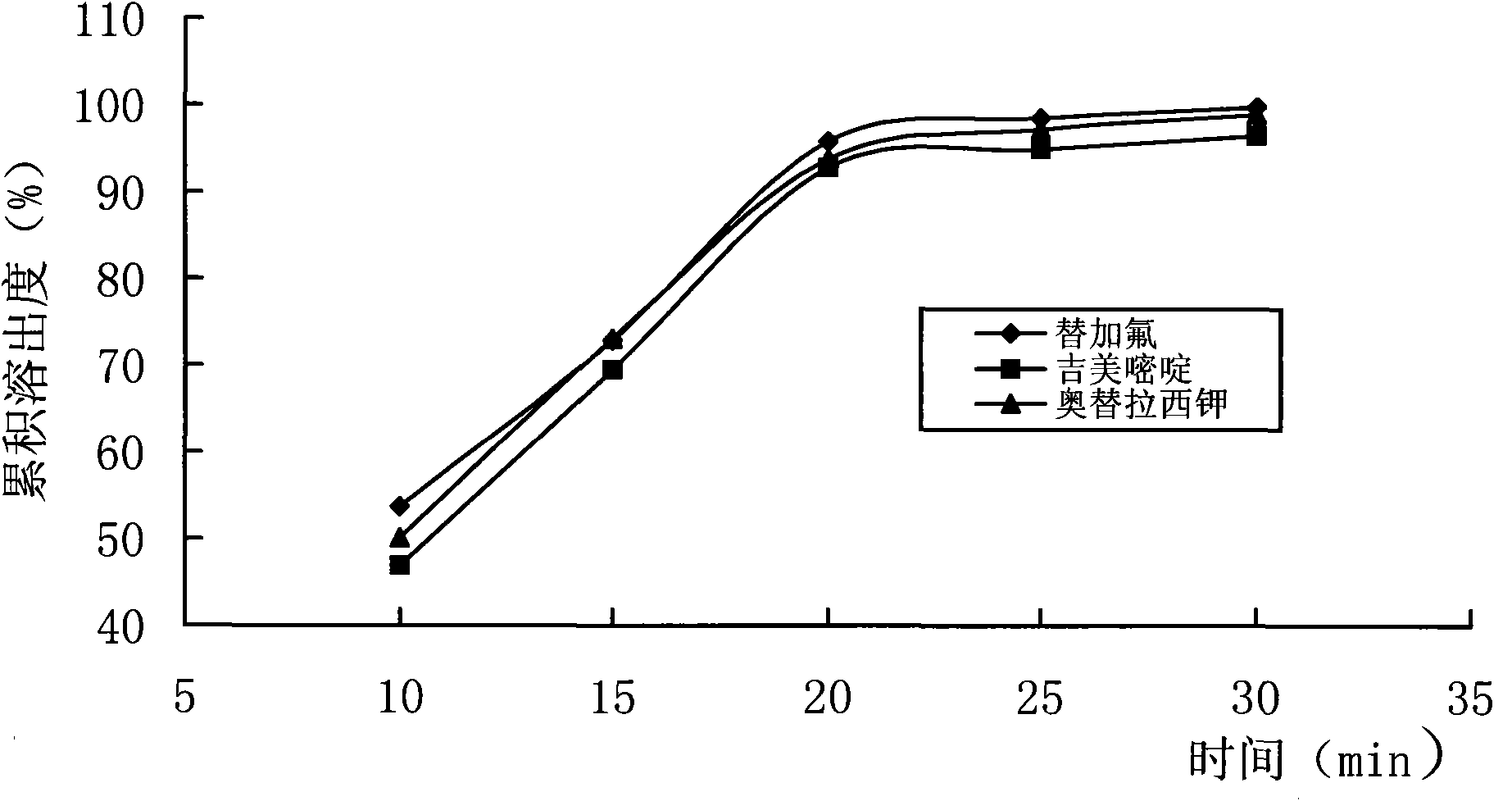
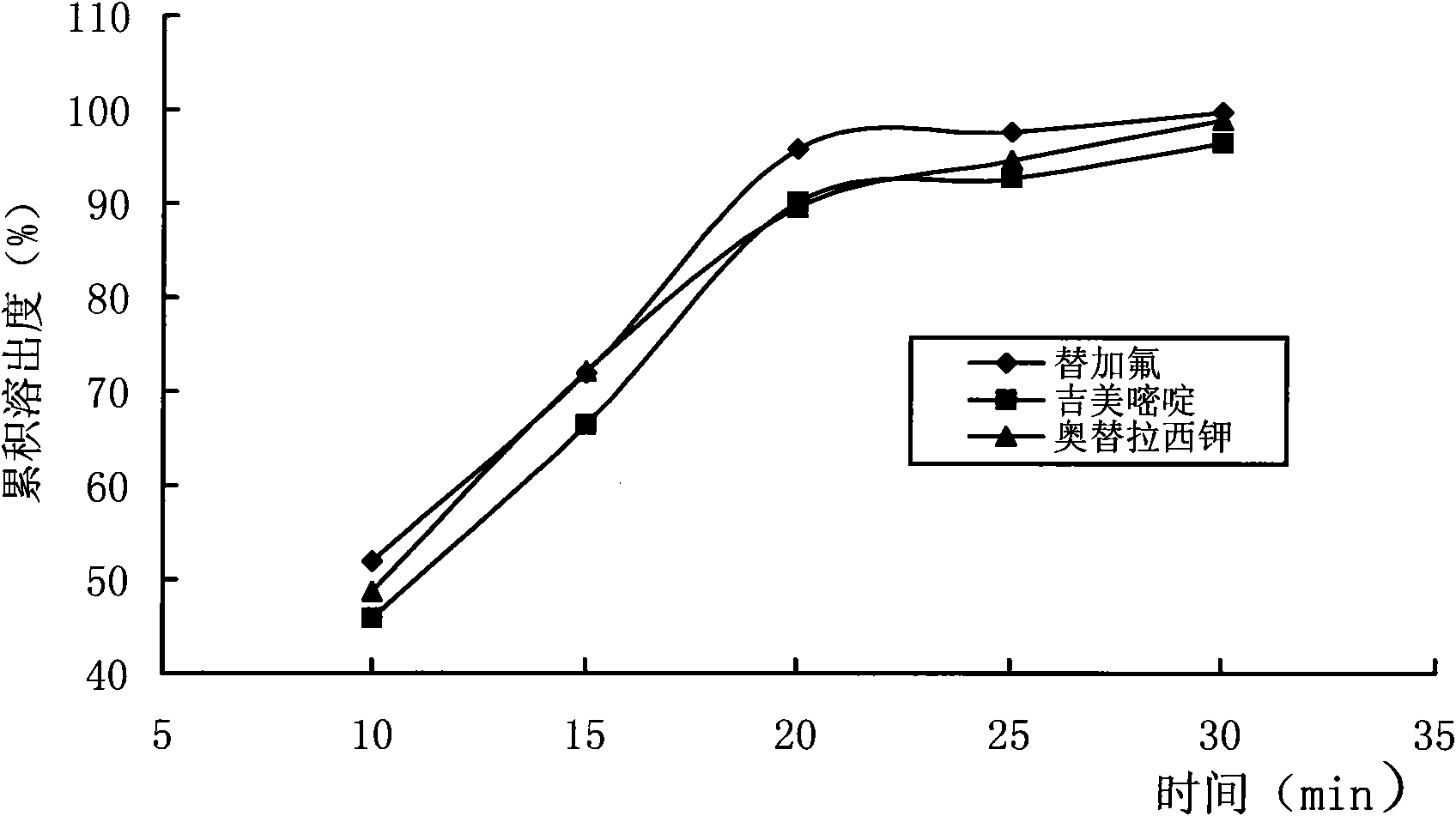
Image
Examples
Embodiment 1
[0041]
[0042] Preparation Process:
[0043] 1) Dry tegafur, gimeracil, oteracil potassium, β-cyclodextrin and other excipients at 40-60°C for 4 hours, pass through a 100-mesh sieve for later use;
[0044] 2) Weigh three parts of β-cyclodextrin according to the prescription amount to prepare saturated aqueous solutions at 45°C, respectively, and then place them in adjustable high-speed mixers, start stirring at a speed of 1000r / min.
[0045] 3) Tegafur, gimeracil and oteracil potassium that take prescription quantity are dissolved in a small amount of 30% acetonitrile aqueous solution respectively, then the solution is slowly added dropwise in the above-mentioned corresponding saturated cyclodextrin aqueous solution, the dropping speed 2ml / min, stirring at constant temperature for 1 hour. Stop heating and continue stirring to room temperature, stop stirring after 4 hours, and spray dry to obtain cyclodextrin inclusion compound.
[0046] (4) Pass the prepared cyclodextrin...
Embodiment 2
[0048]
[0049] Preparation Process:
[0050] 1) Dry tegafur, gimeracil, oteracil potassium, β-cyclodextrin and other excipients at 40-60°C for 4 hours, pass through a 100-mesh sieve for later use;
[0051] 2) Three parts of β-cyclodextrin were weighed according to the prescription amount to make saturated aqueous solutions at 60°C respectively, and then placed in adjustable high-speed mixers respectively, and the stirring was started at a speed of 2000r / min.
[0052] 3) Tegafur, gimeracil and oteracil potassium of prescription quantity are dissolved in a small amount of 30% acetonitrile aqueous solution respectively, then the solution is slowly added dropwise in the above-mentioned corresponding saturated cyclodextrin aqueous solution, and the rate of addition is 3ml / min, stirring at constant temperature for 45min. Stop heating and continue stirring to room temperature, stop stirring after 3.5 hours, and spray dry to obtain β-cyclodextrin inclusion compound.
[0053] 4) ...
Embodiment 3
[0055]
[0056] Preparation Process:
[0057] 1) Dry tegafur, gimeracil, oteracil potassium, α-cyclodextrin and other excipients at 40-60°C for 4 hours, pass through a 100-mesh sieve for later use;
[0058] 2) Weigh three parts of α-cyclodextrin according to the prescription, add appropriate amount of water, grind them to a paste, then pour them into the colloid mill respectively, and turn on the machine.
[0059] 3) Dissolve the prescribed amount of tegafur, gimeracil and oteracil potassium in a small amount of 30% acetonitrile aqueous solution, and then slowly and continuously add the solution dropwise to the above-mentioned corresponding pasty α-cyclodextrin, and grind for 30 minutes , spray-dried to prepare α-cyclodextrin inclusion compound.
[0060] 4) Pass the obtained α-cyclodextrin inclusion compound through a 100-mesh sieve, and then mix it with the remaining auxiliary materials evenly, then use the ethanol solution of hydroxypropyl cellulose to make a soft materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com