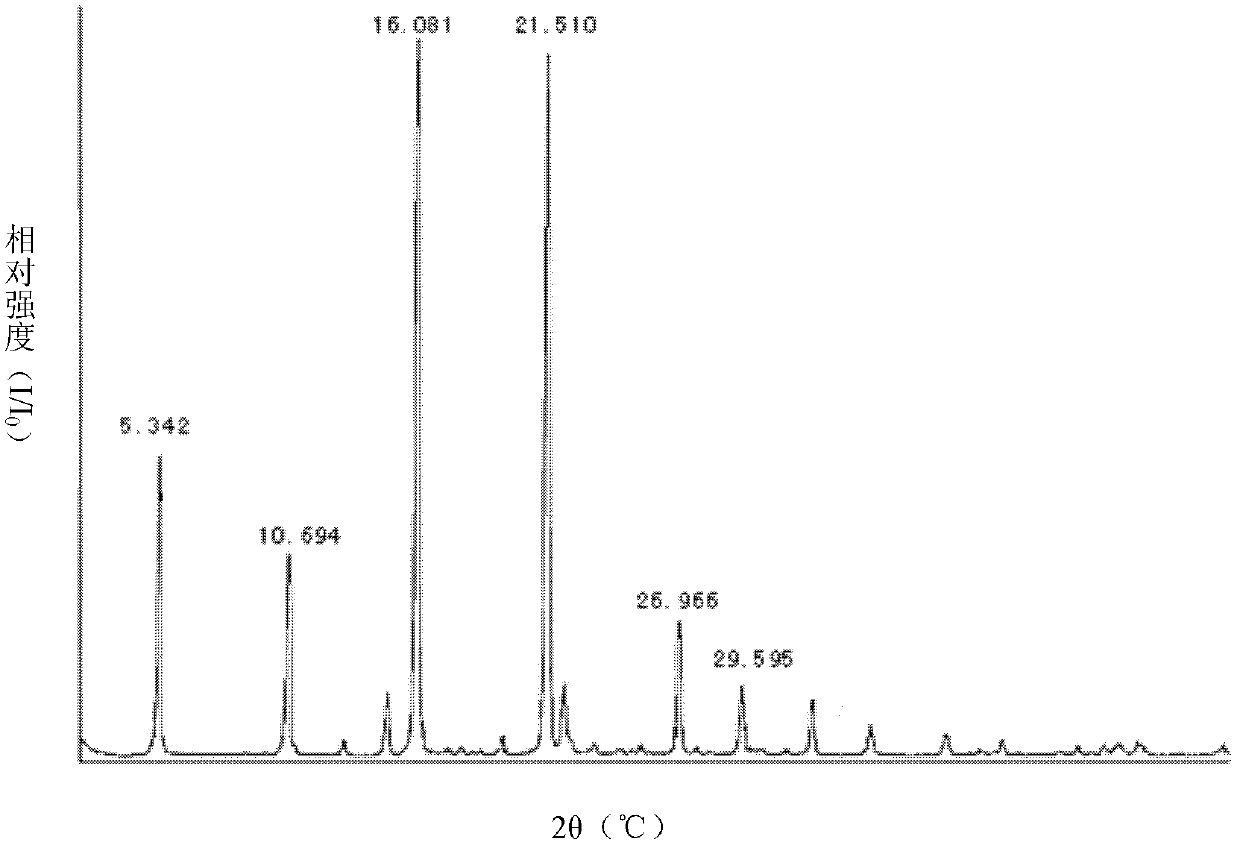
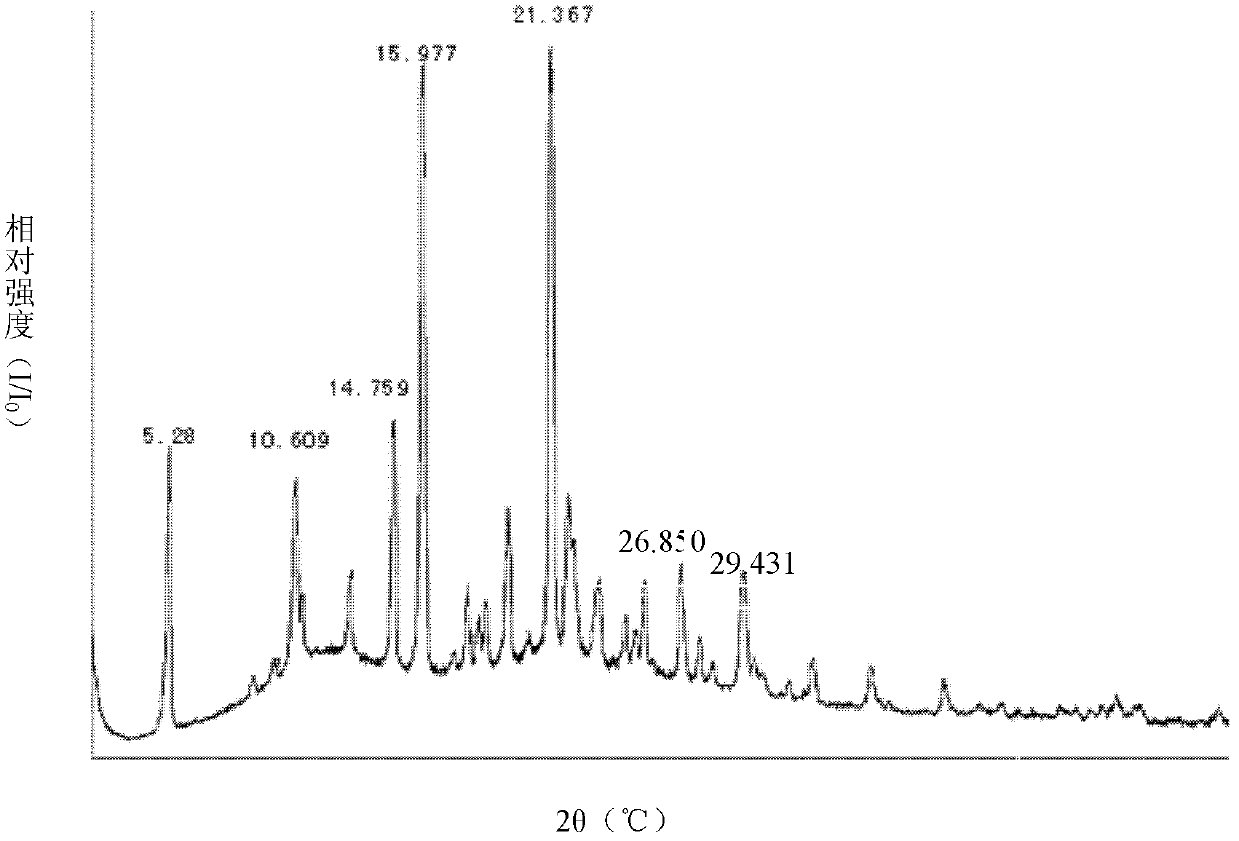
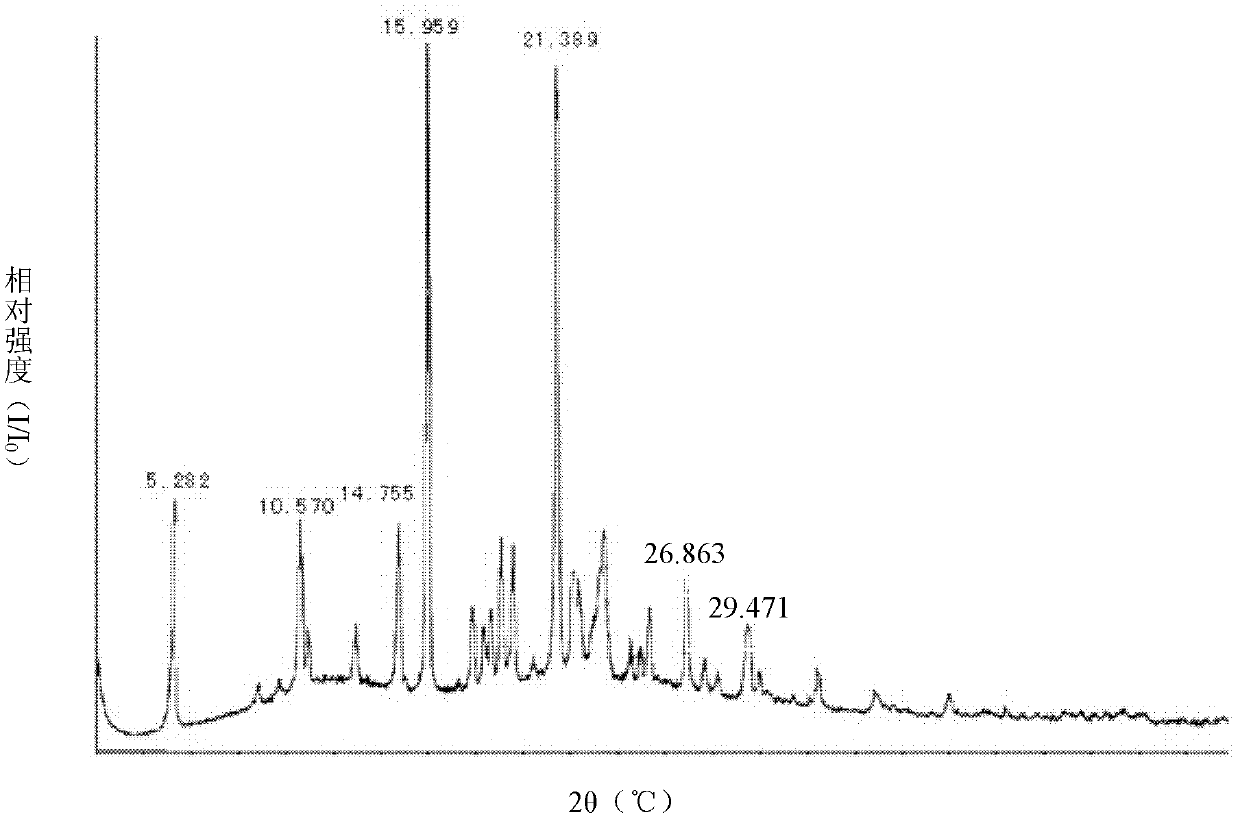
Celecoxib-containing solid dispersion and preparation method thereof
A technology of solid dispersion and celecoxib, which is applied in the field of solid dispersion containing celecoxib and its preparation, can solve the problems of being unsuitable for large-scale industrial production, unsightly appearance of tablets, and reducing the release of the main drug. High brittleness, good fluidity, reducing the effect of drug decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 solid dispersion A
[0042] 100 g of celecoxib and 100 g of copovidone were mixed and placed in a hopper, the temperature of the melting zone was set at 110° C., and the temperature of the extrusion zone was set at 95° C. Start the machine, heat it, and after reaching the set temperature, extrude to obtain solid dispersion A containing celecoxib.
Embodiment 2
[0043] The preparation of embodiment 2 solid dispersion B
[0044] 100 g of celecoxib and 150 g of copovidone were mixed, placed in a hopper, the temperature of the melting zone was set at 110° C., and the temperature of the extrusion zone was set at 95° C. Start the machine, heat it, and after reaching the set temperature, extrude to obtain solid dispersion B containing celecoxib.
Embodiment 3
[0045] The preparation of embodiment 3 solid dispersion C
[0046] 100 g of celecoxib and 300 g of copovidone were mixed and placed in a hopper, the temperature of the melting zone was set at 110° C., and the temperature of the extrusion zone was set at 95° C. Start the machine, heat it, and after reaching the set temperature, extrude to obtain solid dispersion C containing celecoxib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com