Tacrolimus solid dispersion and its preparing method
A technology of solid dispersion and tacrolimus, which is used in medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
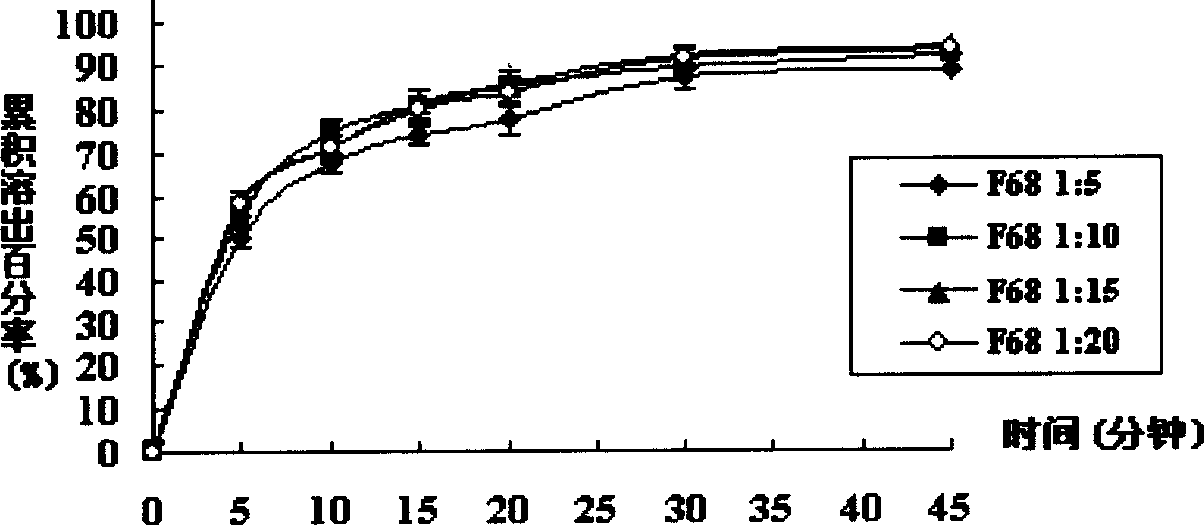
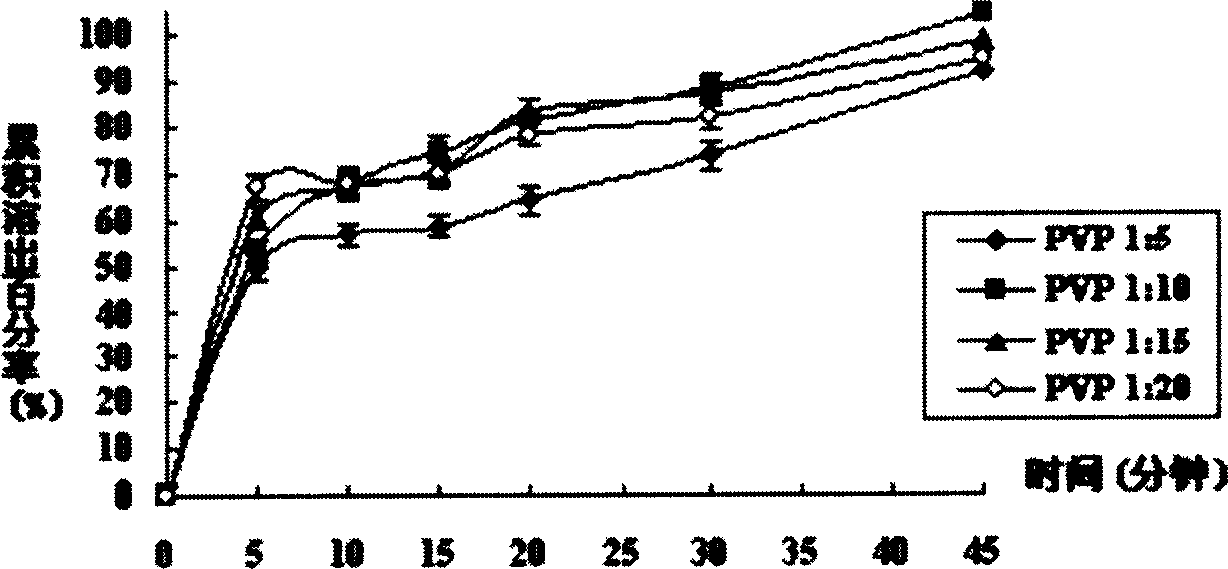
[0030] Take 1g of tacrolimus and 5g of povidone K-29 / 32 into a beaker, add 25mL of absolute ethanol and 25mL of dichloromethane, stir to completely dissolve. In a water bath at 50°C, evaporate with a rotary evaporator for 25 minutes to evaporate the solvent. Transfer to a vacuum drying oven to continue drying for 24 hours (room temperature), take it out, grind, and pass through an 80-mesh sieve to get it. The cumulative in vitro dissolution percentage of the solid dispersion in 45 minutes of the invention is 92.48±1.53% (n=6).
Embodiment 2
[0032] Take 1g of tacrolimus and 10g of povidone K-29 / 32 into a beaker, add 25mL of absolute ethanol and 25mL of dichloromethane, stir to completely dissolve. In a 50°C water bath, evaporate with a rotary evaporator for 25 minutes, and evaporate the solvent to dryness. Transfer to a vacuum drying oven to continue drying for 24 hours (room temperature), take it out, grind, and pass through an 80-mesh sieve to get it. The cumulative in vitro dissolution percentage of the solid dispersion in 45 minutes of the invention is 104.01±5.86% (n=6).
Embodiment 3
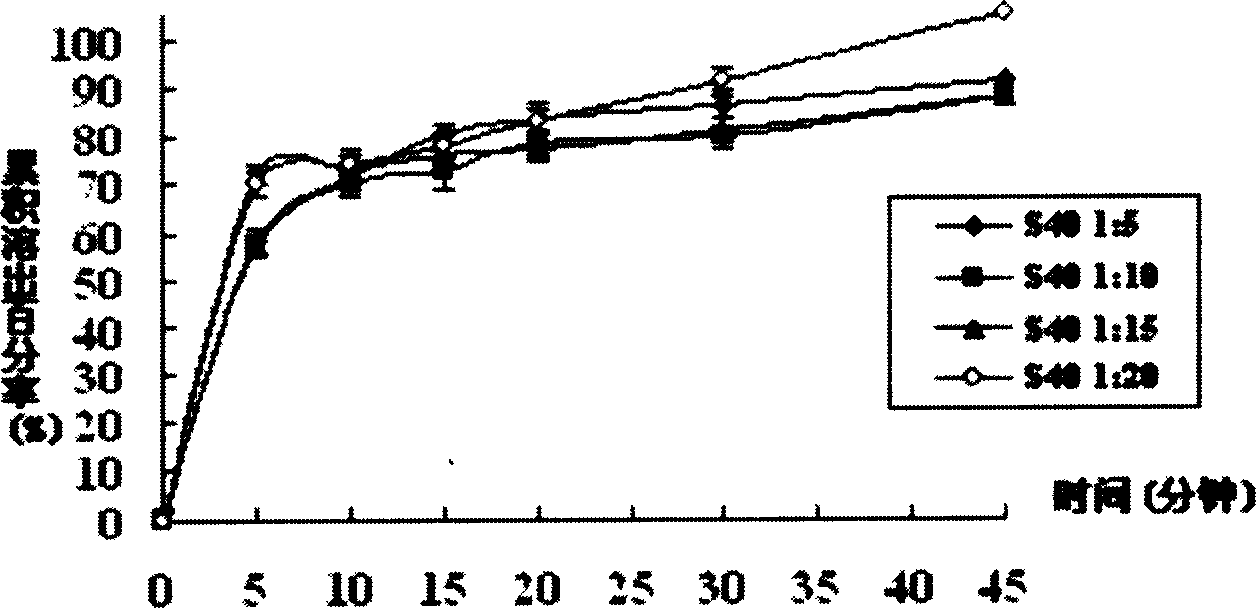
[0034]Take 1g of tacrolimus and 5g of hypromellose E3 into a beaker, add 25mL of absolute ethanol and 25mL of dichloromethane, stir to completely dissolve. In a 50°C water bath, evaporate with a rotary evaporator for 25 minutes, and evaporate the solvent to dryness. Transfer to a vacuum drying oven to continue drying for 24 hours (room temperature), take it out, grind, and pass through an 80-mesh sieve to get it. The cumulative in vitro dissolution percentage of the solid dispersion in 45 minutes of the invention is 103.20±3.28% (n=6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com