Epinastine hydrochloride composition
A technology of epinastine hydrochloride and composition, which is applied in the field of epinastine hydrochloride composition, can solve problems such as difficulty in obtaining uniform mixing, increased difficulty in granulation, unfavorable preparation production, etc., and achieves reducing particle size distribution range, improving Compliance, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
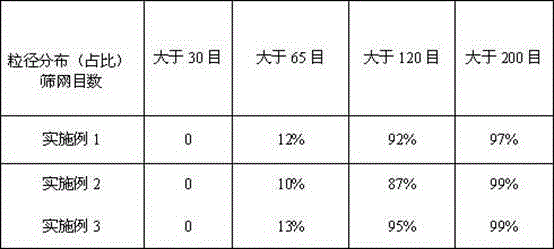
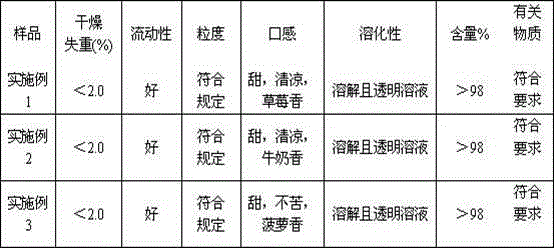
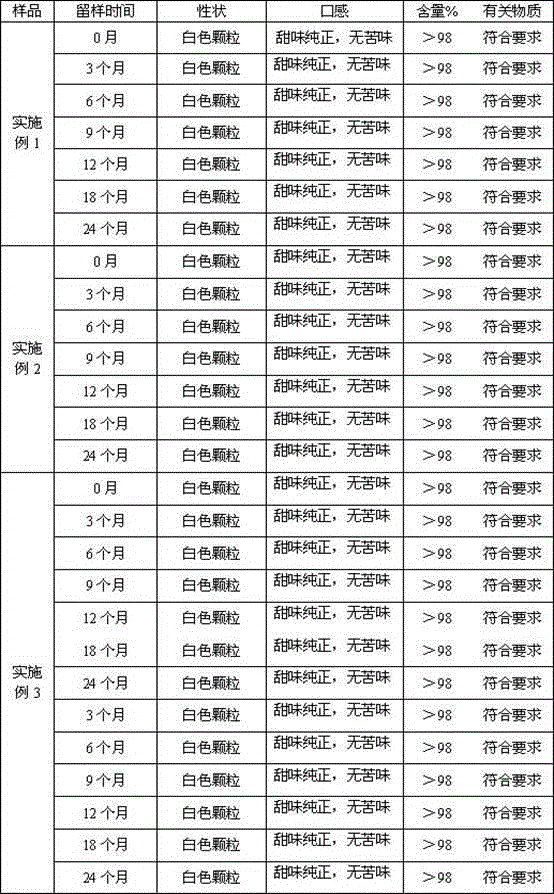
Examples
Embodiment 1
[0045] The composition prescription of fine granule described in the present embodiment is as follows:
[0046] Epinastine Hydrochloride 100g
[0047] Sucrose 9438g
[0048] Aspartame 320g
[0049] Sucralose 40g
[0050] Monoamine Glycyrrhizinate S40g
[0051] Hydroxypropyl Cellulose 42g
[0052] Strawberry essence 20g.
[0053] Firstly, the described epinastine hydrochloride, sucrose, aspartame, sucralose, and monoamine glycyrrhizinate S are respectively passed through a 120 mesh sieve, and are set aside; then the prescribed amount of epinastine hydrochloride and sucrose aspartame Add tannin, sucralose, and monoamine glycyrrhizinate S into the wet granulator, start stirring for 5 minutes until all materials are fully mixed and evenly distributed. Then add 600 g of 5% (w / w) hydroxypropyl cellulose aqueous solution as a binder for granulation. Fluidized bed drying until the water content is less than 3%, sizing with a sieve with a sieve aperture of 0.5mm. Spray 600g of ...
Embodiment 2
[0055] The composition prescription of fine granule described in the present embodiment is as follows:
[0056] Epinastine Hydrochloride 100g
[0057] Erythritol 9438g
[0058] Aspartame 320g
[0059] Sucralose 40g
[0060] Monoamine Glycyrrhizinate S40g
[0061] Hydroxypropyl Cellulose 42g
[0062] 20g of milk essence;
[0063] First described epinastine hydrochloride, erythritol, aspartame, sucralose, glycyrrhizic acid monoamine S are respectively passed through 120 mesh sieves, for subsequent use; then the epinastine hydrochloride and sucrose Put aspartame, sucralose, and monoamine glycyrrhizinate S into the wet granulator, and start stirring for 5 minutes until all materials are fully mixed and uniform. Then add 600 g of 5% (w / w) hydroxypropyl cellulose aqueous solution as a binder for granulation. Fluidized bed drying until the water content is less than 3%, sizing with a sieve with a sieve aperture of 0.5mm. Spray 600g of 2% (w / w) hydroxypropyl cellulose aqueous ...
Embodiment 3
[0065] The composition prescription of fine granule described in the present embodiment is as follows:
[0066] Epinastine Hydrochloride 100g
[0067] Erythritol 4438g
[0068] Sucrose 5000g
[0069] Aspartame 320g
[0070] Sucralose 40g
[0071] Monoamine Glycyrrhizinate S40g
[0072] Hydroxypropyl Cellulose 42g
[0073] 20g pineapple essence;
[0074]First described epinastine hydrochloride, erythritol, sucrose, aspartame, sucralose, glycyrrhizic acid monoamine S are passed through 120 mesh sieves respectively, for subsequent use; Then the epinastine hydrochloride of prescription quantity Put it into the wet granulator with sucrose aspartame, sucralose, and monoamine glycyrrhizinate S, and start stirring for 5 minutes until all materials are fully mixed evenly. Then add 600 g of 5% (w / w) hydroxypropyl cellulose aqueous solution as a binder for granulation. Fluidized bed drying until the water content is less than 3%, sizing with a sieve with a sieve aperture of 0.5mm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com