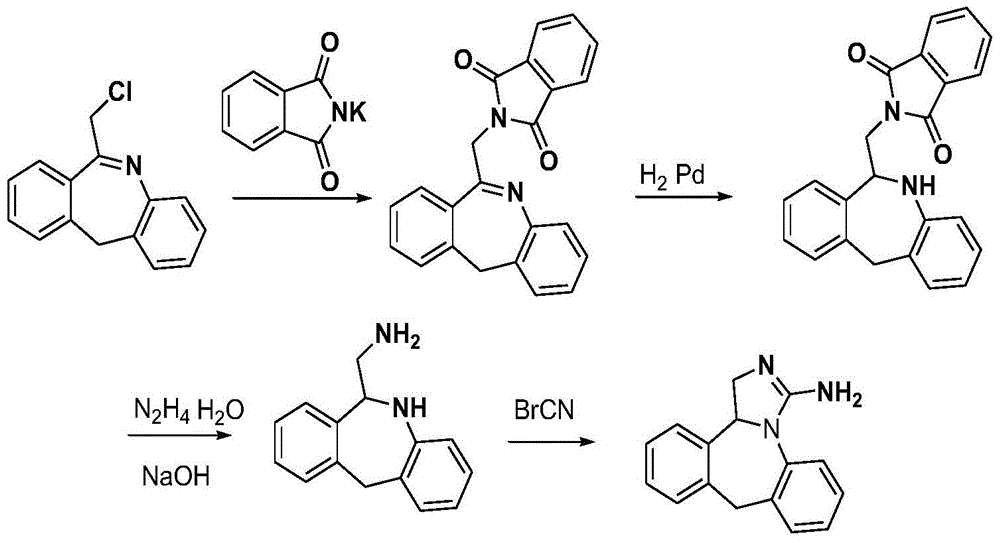
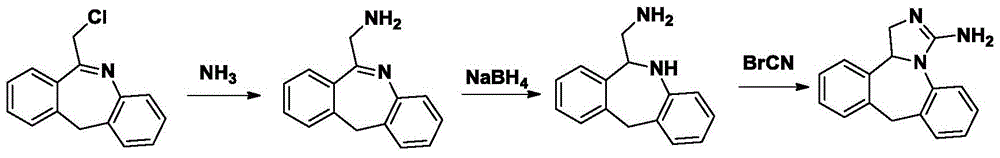
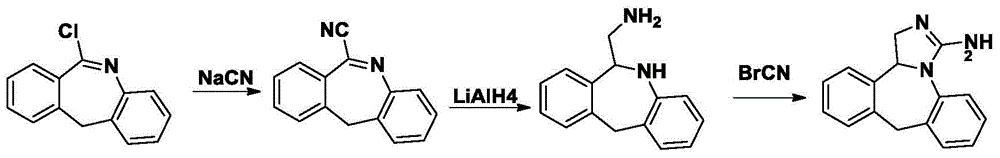Synthesis of Epilastine
A synthetic method and the technology of epinastine, applied in the synthesis of epinastine and the synthesis of histamine H1 receptor antagonists, can solve the problems of unfavorable industrial production, combustion and explosion, etc., and achieve high product yield and good condition Gentle, pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Synthesis of Epinastine
[0036] (1) Synthesis of quaternary ammonium salt
[0037] 6-Chloromethylmorphopidine (24.1g, 0.1mol), urotropine (28g, 0.2mol), and 150ml of chloroform were added to a three-necked flask for reflux reaction for 3 hours, cooled to room temperature, and suction filtered. The filter cake was washed with chloroform, and dried to obtain 30.1 g of quaternary ammonium salt of 6-chloromethylmorphopinidine, with a yield of 79%;
[0038] (2) Synthesis of 6-aminomethylmorphopidine hydrochloride
[0039] Dried 6-chloromethylmorphophene quaternary ammonium salt (25g, 65mmol), methanol 100ml, concentrated hydrochloric acid 12ml, refluxed for 2 hours, cooled to room temperature, suction filtered, and the filtrate recovered ethanol under reduced pressure to obtain 6-aminomethyl Morphenidine hydrochloride 12.9g, yield 76.3%;
[0040] (3) 6-Aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine Synthesis
[0041]6-Aminomethylmorphopidine hydrochloride (...
Embodiment 2
[0046] Example 2 Synthesis of Epinastine
[0047] (1) Synthesis of quaternary ammonium salt
[0048] Add 6-bromomethylmorphopidine (28.5g, 0.1mol), urotropine (28g, 0.2mol), and 150ml of dichloromethane into a three-necked flask, reflux for 3 hours, cool to room temperature, and filter with suction , the filter cake was washed with dichloromethane, and dried to obtain 33.5 g of quaternary ammonium salt of 6-bromomethylmorphopidine, with a yield of 78.8%.
[0049] (2) Synthesis of 6-aminomethylmorphopidine hydrochloride
[0050] Dry 6-bromomethylmorphophene quaternary ammonium salt (30g, 70mmol), 150ml ethanol, 10ml concentrated sulfuric acid, reflux for 2 hours, cool room temperature, filter with suction, and recover ethanol from the filtrate under reduced pressure to obtain 6-aminomethyl Morphenidine hydrochloride 13.9g, yield 76.4%.
[0051] (3) 6-Aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine Synthesis
[0052] 6-Aminomethylmorphopidine hydrochloride (10g, 38mmol) et...
Embodiment 3
[0057] Example 3 Synthesis of Epinastine
[0058] (1) Synthesis of quaternary ammonium salt
[0059] Add 6-bromomethylmorphopidine (0.1mol), urotropine (0.25mol), and 300ml of dichloromethane into a three-necked flask for reflux reaction for 3 hours, cool to room temperature, filter with suction, and filter the cake with dichloromethane Washed with methane and dried to obtain quaternary ammonium salt of 6-bromomethylmorphophenolidinium with a yield of 87.9%.
[0060] (2) Synthesis of 6-aminomethylmorphopidine hydrochloride
[0061] Dry 6-bromomethylmorphophene quaternary ammonium salt (40g, 94mmol), 200ml ethanol, 16ml concentrated hydrochloric acid, reflux for 2 hours, cool to room temperature, filter with suction, and recover ethanol from the filtrate under reduced pressure to obtain 6-aminomethyl Morphenidine hydrochloride 20.1g, yield 82.7%.
[0062] (3) 6-Aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine Synthesis
[0063] 6-Aminomethylmorphopidine hydrochloride (20g,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com