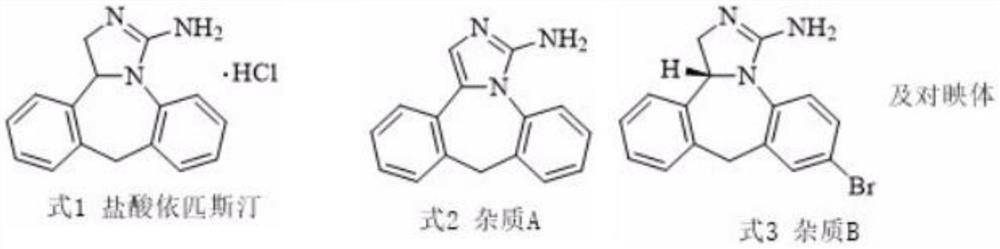
A kind of preparation method of epinastine related substance
A technology for epilastine and related substances, applied in the direction of organic chemistry and the like, can solve the problems of long reaction time, complicated process, low purity epilastine solution and the like, and achieves a simple preparation process, low cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
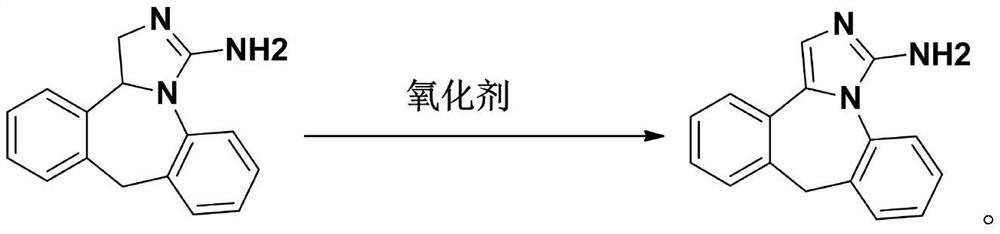
[0023] A preparation method of related substances of epinastine, comprising the steps of:
[0024] Step 1, take 0.01mol epinastine, 12ml N,N-dimethylformamide solution, 0.03mol DBU and 0.011mol CuCl2.2H2O and mix at room temperature for 3 hours;
[0025] Step 2, TLC detects the degree of reaction. After the reaction, add 30ml of water and 60ml of ethyl acetate successively, separate the ethyl acetate layer, extract the water layer with ethyl acetate twice, combine the organic layers and wash with saturated saline three times;
[0026] Step 3, drying with anhydrous sodium sulfate, suction filtration, and recovery of ethyl acetate under reduced pressure;
[0027] Step 4, using n-hexane-ethyl acetate for recrystallization and purification.
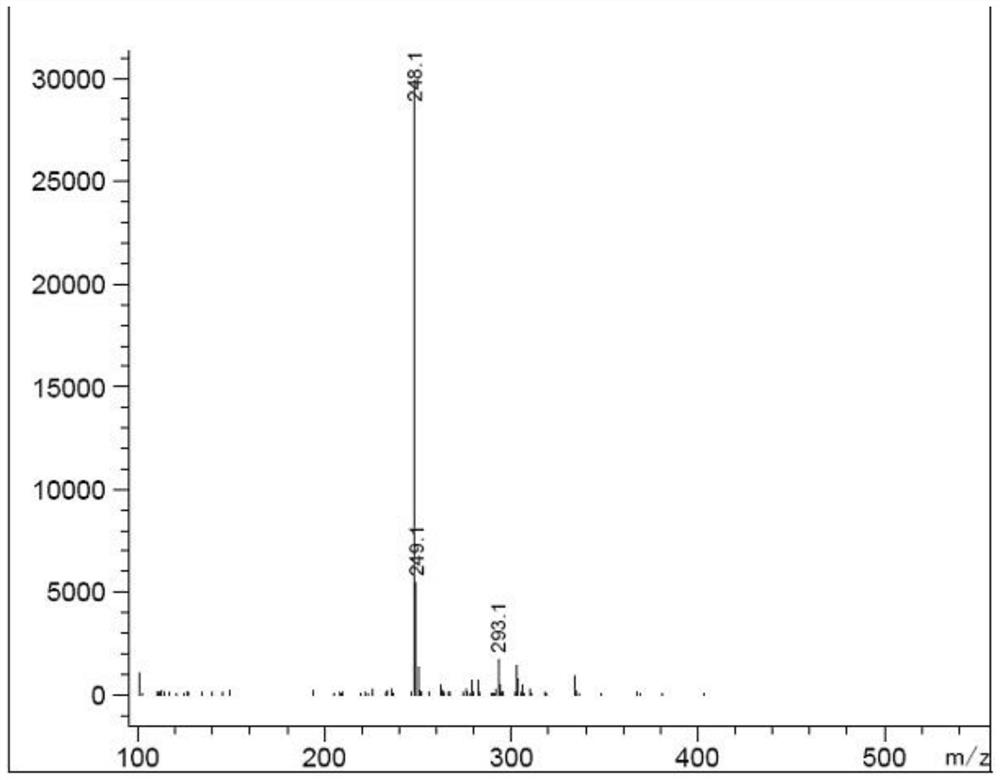
[0028] The above-mentioned recrystallization from n-hexane-ethyl acetate obtained 2.1 g of the epinastine impurity A, the recovery rate was 85%, and the purity of the epinastine impurity A was 97.2%.
[0029] refer to figure 1 Shown, Chemi...
Embodiment 2
[0031] A preparation method of related substances of epinastine, comprising the steps of:
[0032] Step 1, take 0.01mol epinastine, 10ml dimethyl sulfoxide, 1ml hydrochloric acid aqueous solution and 0.011mol ferric chloride and stir at room temperature for 2 hours;
[0033] Step 2, TLC detects the degree of reaction. After the reaction, add 30ml of water and 60ml of ethyl acetate successively, separate the ethyl acetate layer, extract the water layer with ethyl acetate twice, combine the organic layers and wash with saturated saline three times;
[0034] Step 3, drying with anhydrous sodium sulfate, suction filtration, and recovery of ethyl acetate under reduced pressure;
[0035] Step 4, using n-hexane-ethyl acetate for recrystallization and purification.
[0036] Wherein, the hydrochloric acid aqueous solution selects the hydrochloric acid solution that concentration is 10%.
[0037] The above-mentioned recrystallization from n-hexane-ethyl acetate obtained 2.05 g of epin...
Embodiment 3
[0039] Step 1, take 0.01mol epinastine, 20ml N,N-dimethylformamide solution, 0.05mol DBU and 0.02mol CuCl 2 .2H 2 O mixed and stirred at room temperature for 3 hours;
[0040] Step 2, TLC detects the degree of reaction. After the reaction, add 40ml of water and 80ml of ethyl acetate successively, separate the ethyl acetate layer, extract the water layer with ethyl acetate 4 times, combine the organic layers and wash with saturated saline three times;
[0041] Step 3, drying with anhydrous sodium sulfate, suction filtration, and recovery of ethyl acetate under reduced pressure;
[0042] Step 4, using n-hexane-ethyl acetate for recrystallization and purification.
[0043] The above recrystallization through n-hexane-ethyl acetate obtained 1.9 g of epinastine impurity A, the recovery rate was 76.9%, and the purity of epinastine impurity A was 97.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com