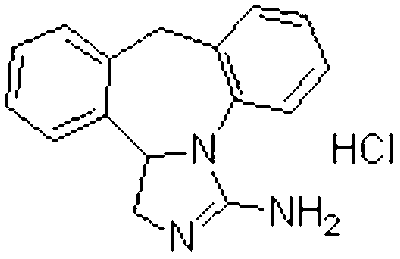
Stable solution containing epinastine or hydrochloride of epinastine
A technology of epilastine and hydrochloride, applied in the field of medical technology preparation, can solve the problems of solution turbidity, reduced curative effect, poor taste, etc., and achieves the effects of easy acceptance, guaranteed safety, and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] formula:
[0030]
[0031] Preparation: Take the raw and auxiliary materials according to the formula, take an appropriate amount of distilled water, stir to dissolve, and adjust the pH with citric acid-sodium citrate buffer solution (take 30g of sodium citrate, add 200ml of water, and adjust the pH value to 5.5±0.2 with sodium hydroxide) When the value reaches 3.0-4.0, add distilled water to dilute to the full amount, filter with a 0.45 μm microporous membrane, fill, stopper, and cap.
Embodiment 2
[0033] formula:
[0034]
[0035] Preparation: Take the raw and auxiliary materials according to the formula, take an appropriate amount of distilled water, stir to dissolve, and adjust the pH with citric acid-sodium citrate buffer solution (take 30g of sodium citrate, add 200ml of water, and adjust the pH value to 5.5±0.2 with sodium hydroxide) When the value reaches 3.0-4.0, add distilled water to dilute to the full amount, filter with a 0.45 μm microporous membrane, fill, stopper, and cap.
Embodiment 3
[0037] formula:
[0038]
[0039] Preparation: Take the raw and auxiliary materials according to the formula, take an appropriate amount of distilled water, stir to dissolve, and adjust the pH with citric acid-sodium citrate buffer solution (take 30g of sodium citrate, add 200ml of water, and adjust the pH value to 5.5±0.2 with sodium hydroxide) When the value reaches 3.0-4.0, add distilled water to dilute to the full amount, filter with a 0.45 μm microporous membrane, fill, stopper, and cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com