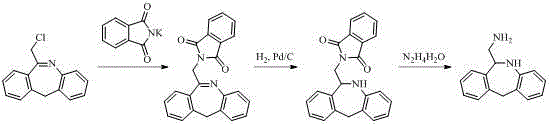
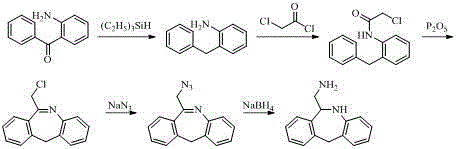
Synthesis method for epinastine intermediate
A synthesis method and technology of borane tetrahydrofuran complexes, which are applied in the field of medicinal chemistry, can solve problems such as unsuitable for industrial production, and achieve the effects of easy industrial production, high yield, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine?
[0030] Add 276ml of borane tetrahydrofuran complex (1mol / L, 0.276mol) into a 1L three-necked flask, stir and cool down to below 0°C in an ice-salt bath. Dissolve 60g (0.276mol) of 6-cyano-11H-dibenzo[b,e]azepine in 120ml of tetrahydrofuran, transfer it to a dropping funnel, and add it slowly, keeping the temperature below 0°C. After the dropwise addition, the temperature was raised to 30-40°C for 2 hours. The ice-salt bath cooled the temperature below 0°C, and slowly added dilute hydrochloric acid dropwise to pH=1 to quench the reaction. After the dropwise addition was completed, stirring was continued for 10 min. Suction filtration, the filter cake was washed with 600ml tetrahydrofuran until it was nearly white, and the filtrate was rotary evaporated under reduced pressure to nearly dryness. Add 300ml of ethyl acetate and 300ml of purified water to the concentrate, stir, and slowly add 30% sodium hy...
Embodiment 2
[0032] Preparation of 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine?
[0033] Add 553ml of borane tetrahydrofuran complex (1 mol / L, 0.553mol) into a 1L three-necked flask, stir and cool down to below 0°C in an ice-salt bath. Dissolve 60g (0.276mol) of 6-cyano-11H-dibenzo[b,e]azepine in 120ml of tetrahydrofuran, transfer it to a dropping funnel, and add it slowly, keeping the temperature below 0°C. After the dropwise addition, the temperature was raised to 30-40°C for 2 hours. The temperature was cooled to 0°C in an ice-salt bath, and dilute hydrochloric acid was slowly added dropwise to pH=1 to quench the reaction. After the dropwise addition was completed, stirring was continued for 10 min. Suction filtration, the filter cake was washed with 600ml tetrahydrofuran until it was nearly white, and the filtrate was rotary evaporated under reduced pressure at 60°C to nearly dryness. Add 300ml of ethyl acetate and 300ml of purified water to the concentrate, stir, and slowly a...
Embodiment 3
[0035] Preparation of 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]azepine?
[0036] Add 1.1L borane tetrahydrofuran complex (1 mol / L, 1.1mol) into a 2L three-necked flask, stir and cool down to below 0°C in an ice-salt bath. Dissolve 60g (0.276mol) of 6-cyano-11H-dibenzo[b,e]azepine in 120ml of tetrahydrofuran, transfer it to a dropping funnel, and add it slowly, keeping the temperature below 0°C. After the dropwise addition, the temperature was raised naturally to 20-30°C for 3 hours. The temperature was cooled to 0°C in an ice-salt bath, and dilute hydrochloric acid was slowly added dropwise to pH=1 to quench the reaction. After the dropwise addition was completed, stirring was continued for 10 min. Suction filtration, the filter cake was washed with 600ml tetrahydrofuran until it was nearly white, and the filtrate was rotary evaporated under reduced pressure at 60°C to nearly dryness. Add 300ml of ethyl acetate and 300ml of purified water to the concentrate, stir, and slow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com