Preparation method for epinastine intermediate
A technology of epilastine and an intermediate, which is applied in the field of preparation of epilastine intermediate N-[2-phenyl]-2-chloroacetamide, can solve the problem that sodium borohydride is expensive, restricts industrial production, and It is not conducive to problems such as industrial production, and achieves the effects of stable and controllable quality, avoiding the introduction of toxic substances and high-boiling solvents, and simplifying the post-processing process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
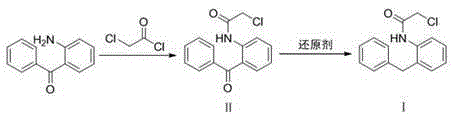
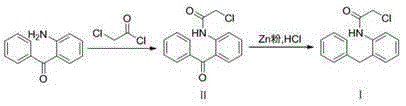
[0025] Dissolve 20 g (0.109 mol) of the raw material 2-aminobenzophenone in 200 mL of dichloromethane, add 9.63 g (0.122 mol) of pyridine, cool down to 0°C, and slowly add 13.17 g (0.117 mol) of chloroacetyl chloride dropwise, After maintaining the reaction at 0°C for 30 min, continue to react at room temperature for 1.5 h, and detect the end point of the reaction by TLC [V (ethyl acetate): V (petroleum ether) = 10:1]. Wash the organic phase with water and dry, filter, retain the filtrate, add 23.21g (0.355mol) of Zn powder, keep the reaction temperature at 0°C, feed HCl gas for 1h while stirring, filter the zinc mud, concentrate the filtrate under reduced pressure, add water to precipitate the solid, pump After filtration and drying, 25.9 g of white solid was obtained, which was Intermediate I, with a yield of 98.6% and a purity of 99.1% by HPLC.
Embodiment 2
[0027] Dissolve 20 g (0.109 mol) of the raw material 2-aminobenzophenone in 200 mL of dichloromethane, add 9.22 g (0.117 mol) of pyridine, cool down to 0°C, and slowly add 12.60 g (0.112 mol) of chloroacetyl chloride dropwise, After maintaining the reaction at 0°C for 30 minutes, continue to react at room temperature for 2 hours, and detect the end point of the reaction by TLC [V (ethyl acetate): V (petroleum ether) = 10:1]. Wash the organic phase with water and dry it, filter it, and evaporate the solvent to dryness to obtain intermediate II. Dissolve it in 200 mL of ether, add 9.95 g (0.152 mol) of Zn powder, keep the reaction temperature at 10°C, and inject HCl gas for 2 h while stirring , filtered the zinc mud, concentrated the filtrate under reduced pressure, added water to precipitate the solid, filtered it with suction, and dried it to obtain 22.5 g of a white solid, namely Intermediate I, with a yield of 85.3% and a purity of 90.5% by HPLC.
Embodiment 3
[0029] Dissolve 20 g (0.109 mol) of the raw material 2-aminobenzophenone in 200 mL of dichloromethane, add 9.63 g (0.122 mol) of pyridine, cool down to 0°C, and slowly add 12.60 g (0.112 mol) of chloroacetyl chloride dropwise, After maintaining the reaction at 0°C for 30 minutes, continue to react at room temperature for 2 hours, and detect the end point of the reaction by TLC [V (ethyl acetate): V (petroleum ether) = 10:1]. Wash the organic phase with water and dry it, filter it, and evaporate the solvent to dryness to obtain intermediate II, which is dissolved in 200 mL of tetrahydrofuran, and 29.84 g (0.456 mol) of Zn powder is added. The reaction temperature is maintained at -10 ° C, and HCl gas is introduced under stirring After 1 hour, the zinc mud was filtered, the filtrate was concentrated under reduced pressure, the solid was precipitated by adding water, suction filtered, and dried to obtain 25.08 g of a white solid, namely Intermediate I, with a yield of 95.2% and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com