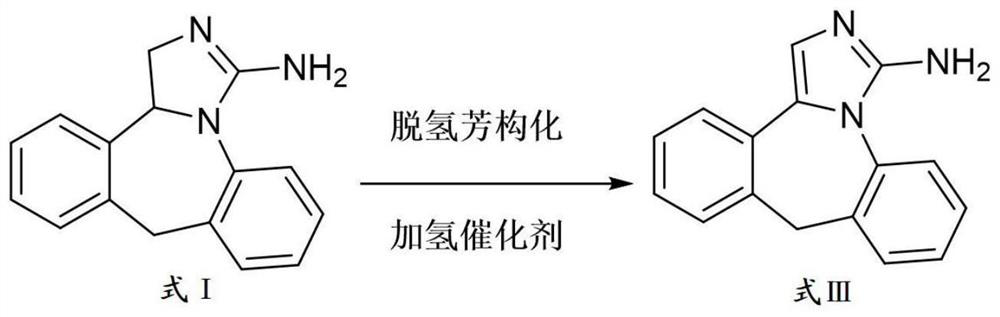
Preparation method of epinastine impurity A
A technology of epinastine and impurity, which is applied in the field of preparation of epinastine impurity A, can solve the problems of epinastine impurity A, such as low purity, low reaction conversion rate, and many side reactions, and achieve simple process, The effect of high purity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] The embodiment of the present application provides a kind of epinastine impurity A, and its preparation method comprises:
[0038] Step 1: Add 7.9g of epinastine free base, 6.0g of Pd / C, 200mL of toluene into the reaction flask, heat to 110°C and reflux for 26h to prepare reactant 1.
[0039] Step 2: The reactant 1 in step 1 is filtered and washed in sequence to obtain a substance after removing Pd / C, which is then concentrated to obtain reactant 2.
[0040] Step 3: Add the reactant 2 of step 2 into acetone to make a slurry, filter and then vacuum-dry to obtain an off-white solid product.
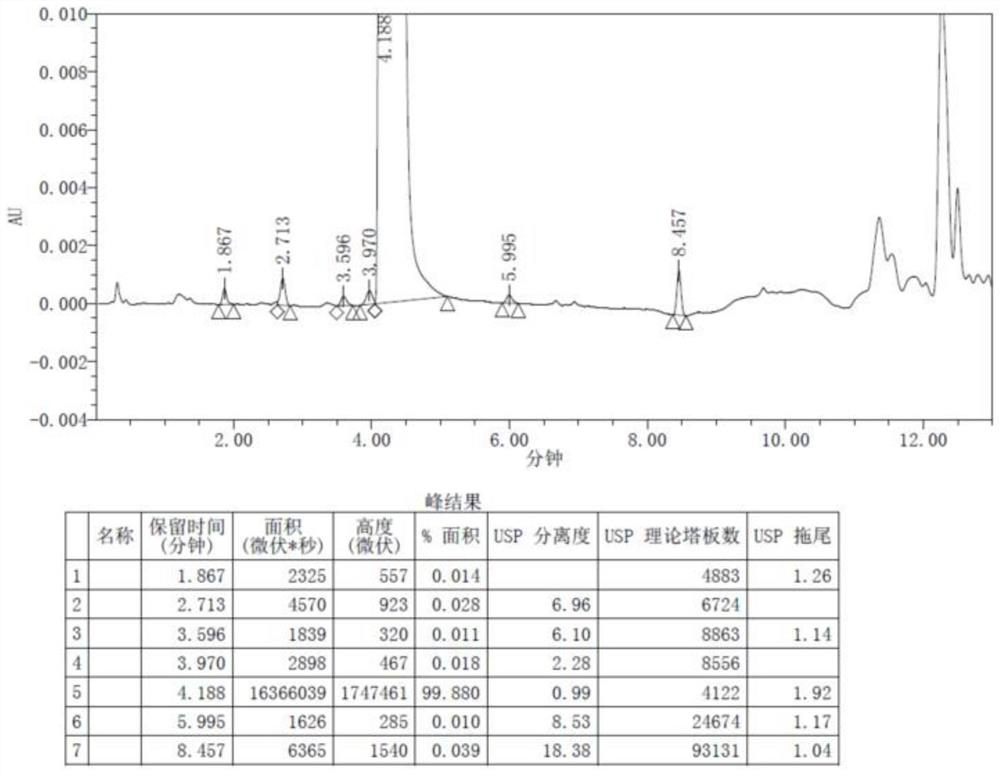
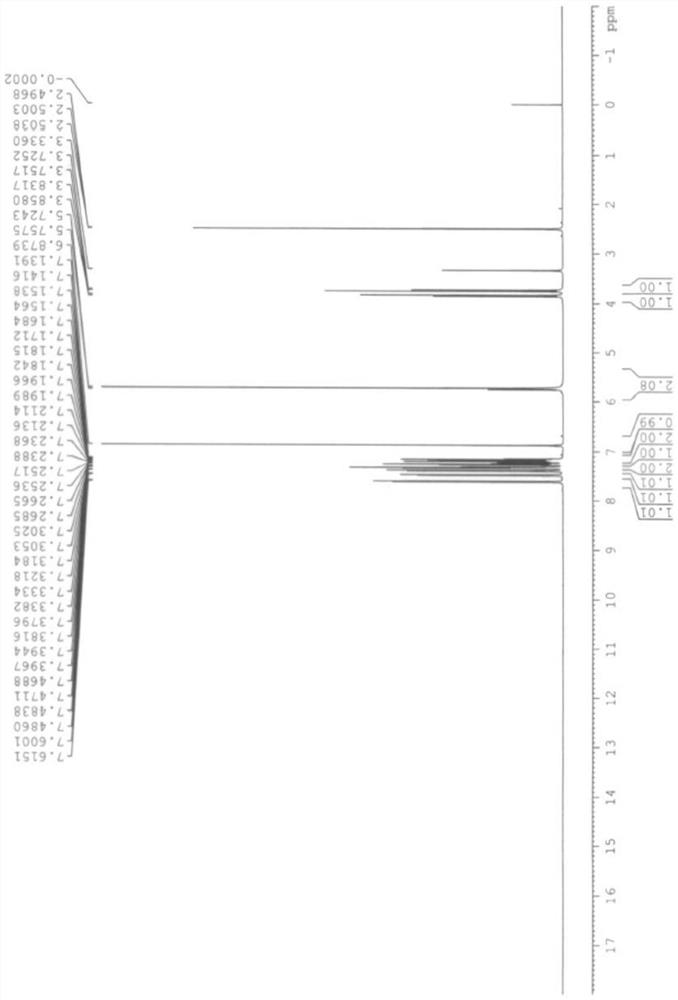
[0041] The product of the embodiment of the present application is carried out HPLC spectrogram, proton nuclear magnetic spectrum spectrogram and mass spectrogram analysis, the result is as follows Figure 1 ~ Figure 3 shown. from Figure 1 ~ Figure 3 It can be seen that the product obtained in the examples of the present application is epinastine impurity A. The mass of epinasti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com