Stable granular medicine composition containing epinastine or hydrochloride thereof
A technology of epinastine and composition, applied in the field of pharmaceutical technology preparation
Inactive Publication Date: 2013-08-21
BEIJING KEYUAN CHUANGXIN TECH
View PDF3 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In the instruction sheet of epinastine hydrochloride granules published by Japan’s independent administrative body Pharmaceuticals and Medical Devices Comprehensive Management Agency, it is mentioned that its prescription consists of erythritol, aspartame, hydroxypropyl cellulose, sodium saccharin, di Silicon oxide, monoammonium glycyrrhizinate, fumaric acid, disodium hydrogen phosphate, essence, etc., but there is no description or hint when epinastine hydrochloride is prepared by a common preparation method, in the prepared preparation, Epinastine hydrochloride, the active ingredient, decomposes significantly over time
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0027]
Embodiment 2
[0029]
Embodiment 3
[0031]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a stable granular medicine composition containing epinastine or hydrochloride thereof. The stable granular medicine composition also comprises a binding agent which has a stabilizing effect on the epinastine or the hydrochloride thereof. Meanwhile, the invention also discloses a preparation method of the stable granular medicine composition. The granular medicine composition disclosed by the invention can also be used as a medicament which urgently needs to be developed in clinic and has an excellent effect on allergic reaction diseases of children.
Description
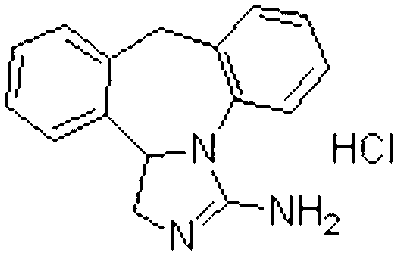
technical field [0001] The invention belongs to the field of pharmaceutical technology preparation, and in particular relates to a stable granular pharmaceutical composition formed by adopting epinastine or its hydrochloride and a specific binder and a preparation method thereof. Background technique [0002] Epinastine is as shown in the following structural formula (I): [0003] [0004] Structural formula (I) It is chemically known as 3-amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine hydrochloride. It is reported that a series of substances, including epinastine or its hydrochloride, have highly selective affinity for H1 receptors, and have strong antagonistic effects on histamine, leukotriene C4, PAF, serotonin, etc. And can inhibit the release of chemical mediators such as histamine and SRS-A (slow reacting substance A). [0005] The granular composition involved in the present invention is clinically used for the treatment of children's allergic rhinitis,...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/16A61K31/55A61K47/34A61P11/02A61P17/00A61P17/04A61P37/08
Inventor 李晓红
Owner BEIJING KEYUAN CHUANGXIN TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com