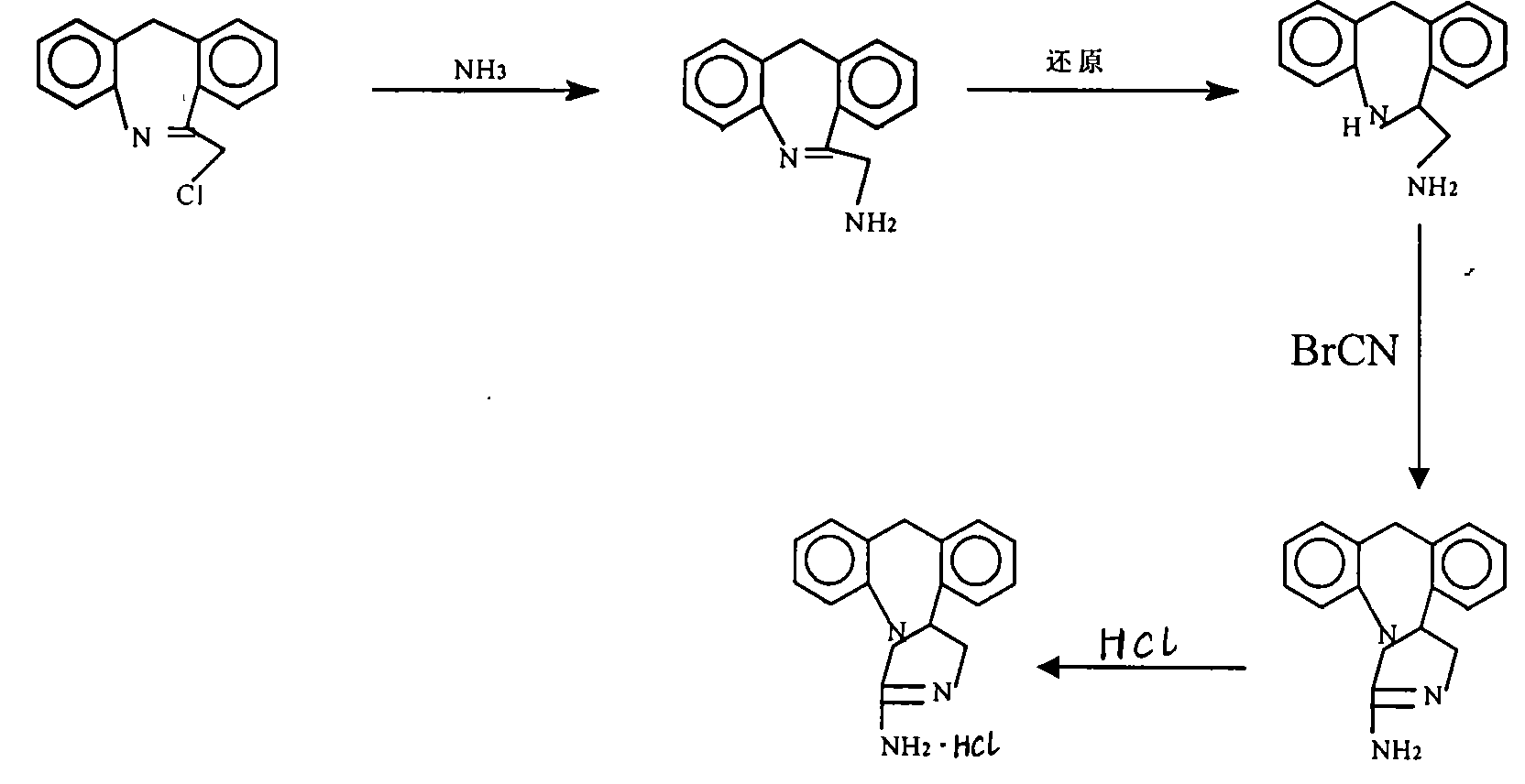
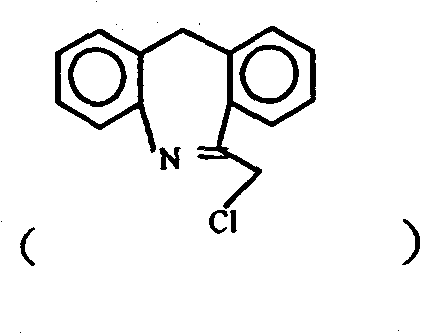
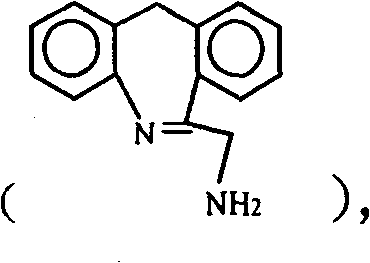
Chemical synthesis method for epinastine
A chemical synthesis, epinastine technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems that are not suitable for industrial production, and achieve high reaction yield, less by-products, and simple preparation Effect
Inactive Publication Date: 2010-09-08
HANGZHOU LONGSHAN CHEM CO LTD
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a new chemical synthesizing method of yipisidin, which comprises the following steps: ammonifying 6-chloromethyl-11-dihyrogen-dibenz [b,e] aza to generate 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza; reducing the 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza into 6-aminomethyl-6,11-dihydrogen-5H-dibenz [b,e] aza; generating the product through cyanogen bromide to loop. Theinvention simplifies the making method with little by-product, which improves the receiving rate by 69% with high purity (HPLC. 99. 0%) for industrial manufacturing.
Description
technical field The invention relates to a novel chemical synthesis method of epinastine. It specifically relates to a method for synthesizing epinastine by using 6-chloromethyl-11-dihydro-dibenzo[b,e]azepine as a starting material through ammoniation reaction, reduction reaction and ring closure reaction . Starting material 6-chloromethyl-11-dihydro-dibenzo [b, e] azepine can be according to J.Am.Chem.Soc. Magazine 1970, Vol.13, P35 published literature method prepared. Background technique Epinastine is suitable for skin allergic reactions and has good curative effect on allergic rhinitis and allergic bronchial asthma. Wide range of clinical application departments. It has high selectivity and affinity for H1 receptors, and its anti-allergic effect is stronger than that of traditional drugs. With multiple anti-allergic mechanisms, it can eliminate allergic symptoms more thoroughly. The onset of effect is more rapid, and the pain of patients can be relieved quickly. ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D487/04A61K31/55A61P37/08
Inventor 王彬峰谭忠宇
Owner HANGZHOU LONGSHAN CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com