Synthesis method of epinastine
A synthesis method and the technology of Epilastine, applied in the field of medicinal chemistry, can solve the problems of high equipment requirements, unfavorable industrialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
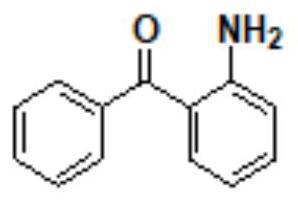
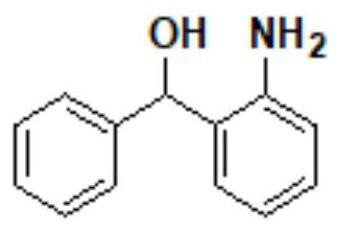
[0103] Step 1: Synthesis of (2-aminophenyl) (phenyl) methanol (compound II)
[0104] Add 60g of compound I 2-aminobenzophenone and 300g of 95% ethanol solution into the clean and anhydrous first reaction flask, then slowly add 6g of sodium borohydride into the first reaction flask, and add the solution in the first reaction flask to the first reaction flask. The temperature of the first reaction flask was slowly raised to 75~80℃ for 2.0h, and the temperature of the solution in the first reaction flask was 75~80℃ and the air pressure was -0.09MPa, which was concentrated to 95% ethanol in the solution in the first reaction flask. The solution was removed, 300 g of water was added to the concentrated first reaction flask while it was still hot, stirred for 1 h to reduce the temperature of the solution in the first reaction flask to 10-15 °C, suction filtration, and drying to obtain compound II. In one step, the molar yield of compound II was 99.81%, and the HPLC purity was 99.867...
Embodiment 2
[0120] Step 1: Synthesis of (2-aminophenyl) (phenyl) methanol (compound II)
[0121] The air pressure was -0.05MPa, and the rest was the same as that of step 1 in Example 1. The molar yield of compound II was 99.65%, and the HPLC purity was 99.923%.
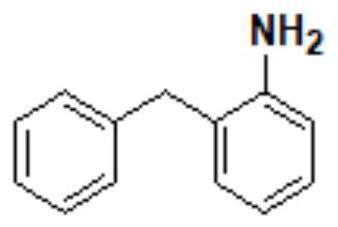
[0122] Step 2: Synthesis of 2-benzylaniline (compound III)
[0123] The air pressure is -0.05MPa, and the rest are the same as the second step of the first embodiment.
[0124] Step 3: Synthesis of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide (Compound IV)
[0125] The air pressure was -0.05MPa, and the rest were the same as in step 3 of Example 1. The molar yield of compound IV was 90.27%, and the HPLC purity was 99.176%.
[0126] Step 4: Synthesis of 6-Chloromethyl Morphenidine (Compound V)
[0127] The air pressure is -0.05MPa, and the rest are the same as in Step 4 of Example 1.
[0128] Step 5: Synthesis of 6-(phthalimidomethyl)-6,11-dihydro-dibenzo-[b,e]azepine (Compound VI)
[0129] Add 140g DMF, 63.8g potassium carbo...
Embodiment 3
[0136] Step 1: Synthesis of (2-aminophenyl) (phenyl) methanol (compound II)
[0137] The air pressure was -0.07MPa, and the rest was the same as that of step 1 in Example 1. The molar yield of compound II was 97.83%, and the HPLC purity was 99.785%.
[0138] Step 2: Synthesis of 2-benzylaniline (compound III)
[0139] Add 59.3g of compound II and 240g of tetrahydrofuran to the clean and anhydrous second reaction flask, control the temperature of the solution in the second reaction flask at 0-10°C and add 72.28g of aluminum trichloride, 8.5 g of aluminum trichloride to the second reaction flask in turn. g sodium borohydride, and then the temperature of the solution in the second reaction flask was raised to 60-70 °C, and the reaction was refluxed for 12.0 h, under the conditions that the temperature of the solution in the second reaction flask was 60-70 °C and the air pressure was -0.07MPa Concentrate until the tetrahydrofuran in the solution in the second reaction bottle is r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com