A kind of synthetic method of epinastine hydrochloride
A technology of epilastine hydrochloride and a synthesis method, which is applied in the field of chemical substance synthesis, and can solve problems such as difficulty in obtaining starting materials, unfavorable industrial production, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
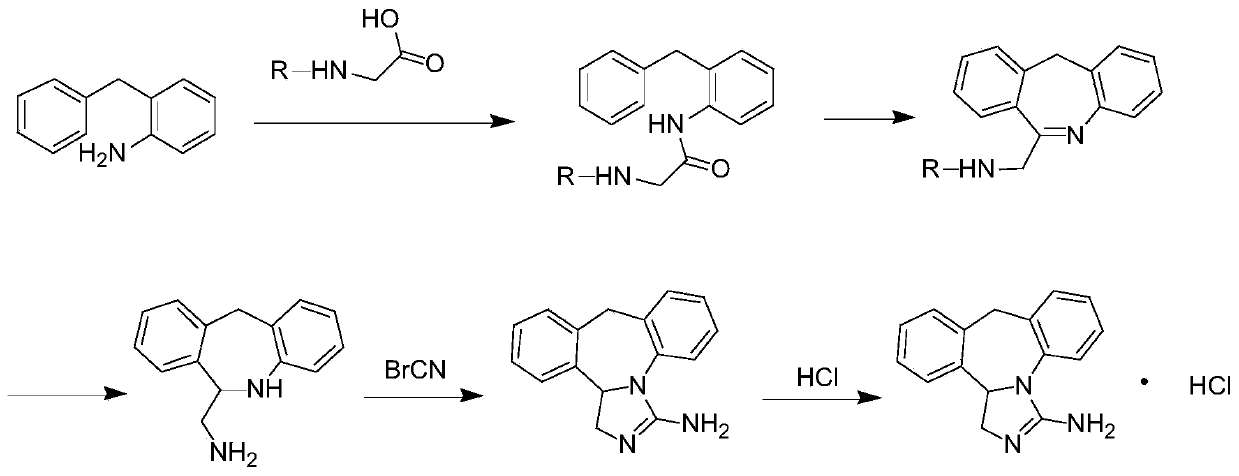
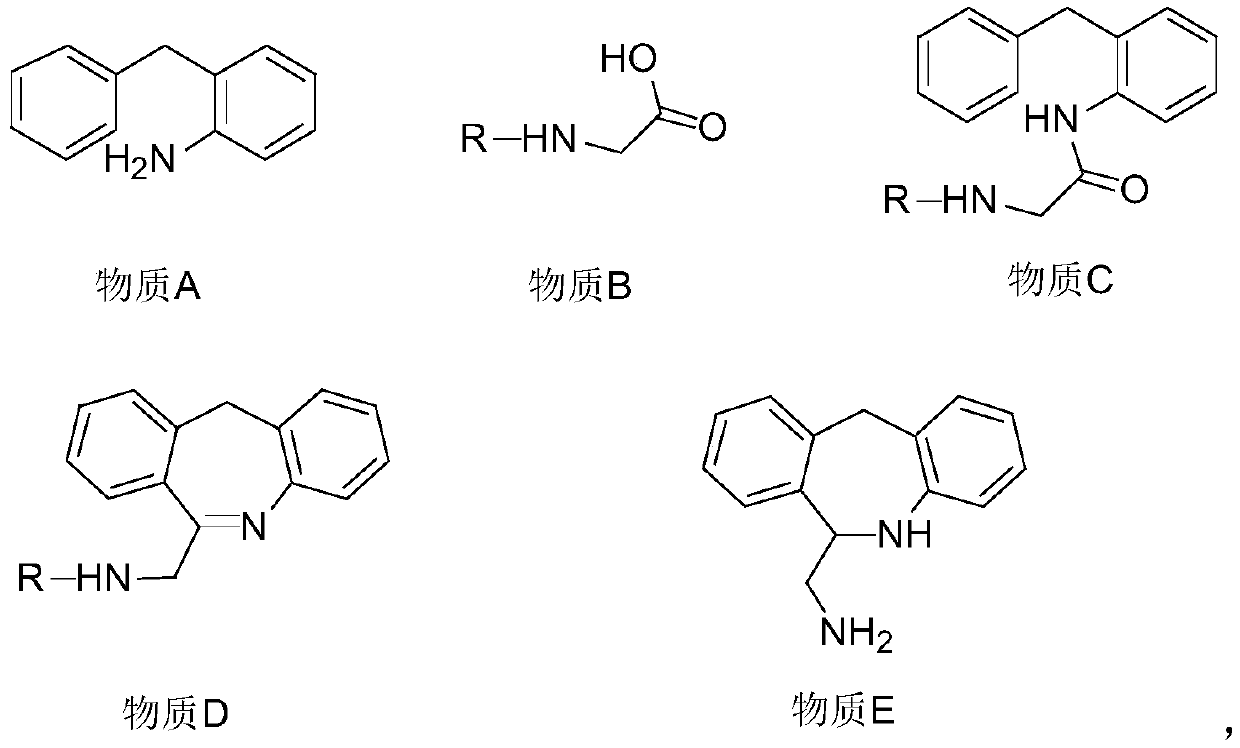
[0094] A method for synthesizing epinastine hydrochloride, including the following steps: mixing tert-butoxycarbonyl protected substance B with dichloromethane, adjusting the temperature to 15°C, adding dicyclohexylcarbodiimide, and stirring for 0.5h, Then add the substance A dichloromethane solution dropwise, keep stirring for 12h, add water, 3wt% potassium carbonate aqueous solution and saturated sodium chloride aqueous solution in turn, and wash once. Take the organic layer for each extraction and then concentrate under reduced pressure. Drying to obtain substance C, wherein the molar ratio of substance A to substance B is 1:1.2, and the weight ratio of substance B to dicyclohexylcarbodiimide is 1:1;
[0095] Mix substance C with polyphosphoric acid, heat to 120°C, keep stirring for 2h, add dichloromethane to extract the organic layer, wash the organic layer with water, 3wt% potassium carbonate aqueous solution and saturated sodium chloride aqueous solution successively, reduce...
Embodiment 2
[0099] A method for synthesizing epinastine hydrochloride, including the following steps: mix benzyl protected substance B with ethyl acetate, adjust the temperature to 35°C, add 4,5-dicyanoimidazole, stir for 1 hour, and then drop Add substance A ethyl acetate solution, keep warm and stir for 2h, add water, mass fraction of 7wt% sodium carbonate aqueous solution, saturated sodium chloride aqueous solution each extraction and wash once, take the organic layer for each extraction, then concentrate under reduced pressure and dry Obtain substance C, wherein the molar ratio of substance A to substance B is 1:1, and the weight ratio of substance B to 4,5-dicyanoimidazole is 1:1.2;
[0100] Mix substance C with polyphosphoric acid, heat to 160°C, keep stirring for 0.5h, add ethyl acetate to extract the organic layer, wash the organic layer with water, 7wt% sodium carbonate aqueous solution and saturated sodium chloride aqueous solution in turn, Concentrate under reduced pressure and dr...
Embodiment 3
[0104] A method for synthesizing epinastine hydrochloride, comprising the following steps: mixing the benzyloxycarbonyl protected substance B with dichloromethane, adjusting the temperature to 20°C, adding N,N-carbonyl-diimidazole, and stirring for 0.6h, Then add dropwise the 1,2-dichloroethane solution of substance A, keep it warm and stir for 10 hours, add water, 4wt% sodium hydroxide aqueous solution and saturated sodium chloride aqueous solution successively and wash once, each extraction washes organic Layer, then concentrated under reduced pressure and dried to obtain substance C, wherein the molar ratio of substance A to substance B is 1:1.18, and the weight ratio of substance B to N,N-carbonyl-diimidazole is 1:1.13;
[0105] Mix substance C with polyphosphoric acid, heat to 130°C, keep stirring for 1.5h, add dichloromethane to extract the organic layer, and wash the organic layer with water, 4wt% sodium hydroxide aqueous solution and saturated sodium chloride aqueous solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com