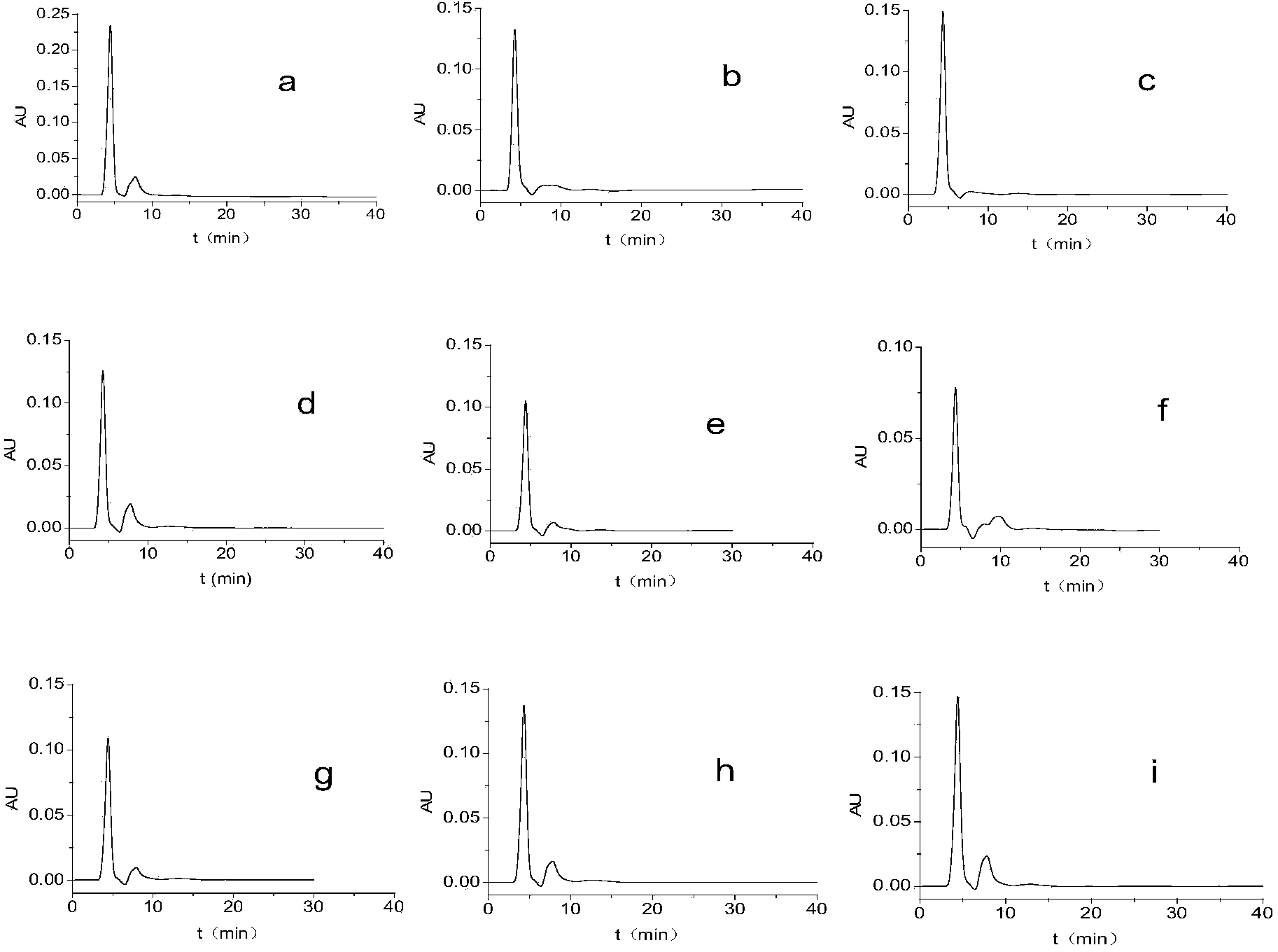Bis-(6-oxo-m-nitrobenzaldehyde sulfonyl)-beta-cyclodextrin as well as preparation method and application thereof
A technology of nitrobenzenesulfonyl and nitrobenzenesulfonyl chloride is applied in the field of preparation and application of chiral selectors, and can solve the problems of not establishing separation and detection methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] β-CD-N 2 Preparation and application effect of chiral HPCE monolithic column
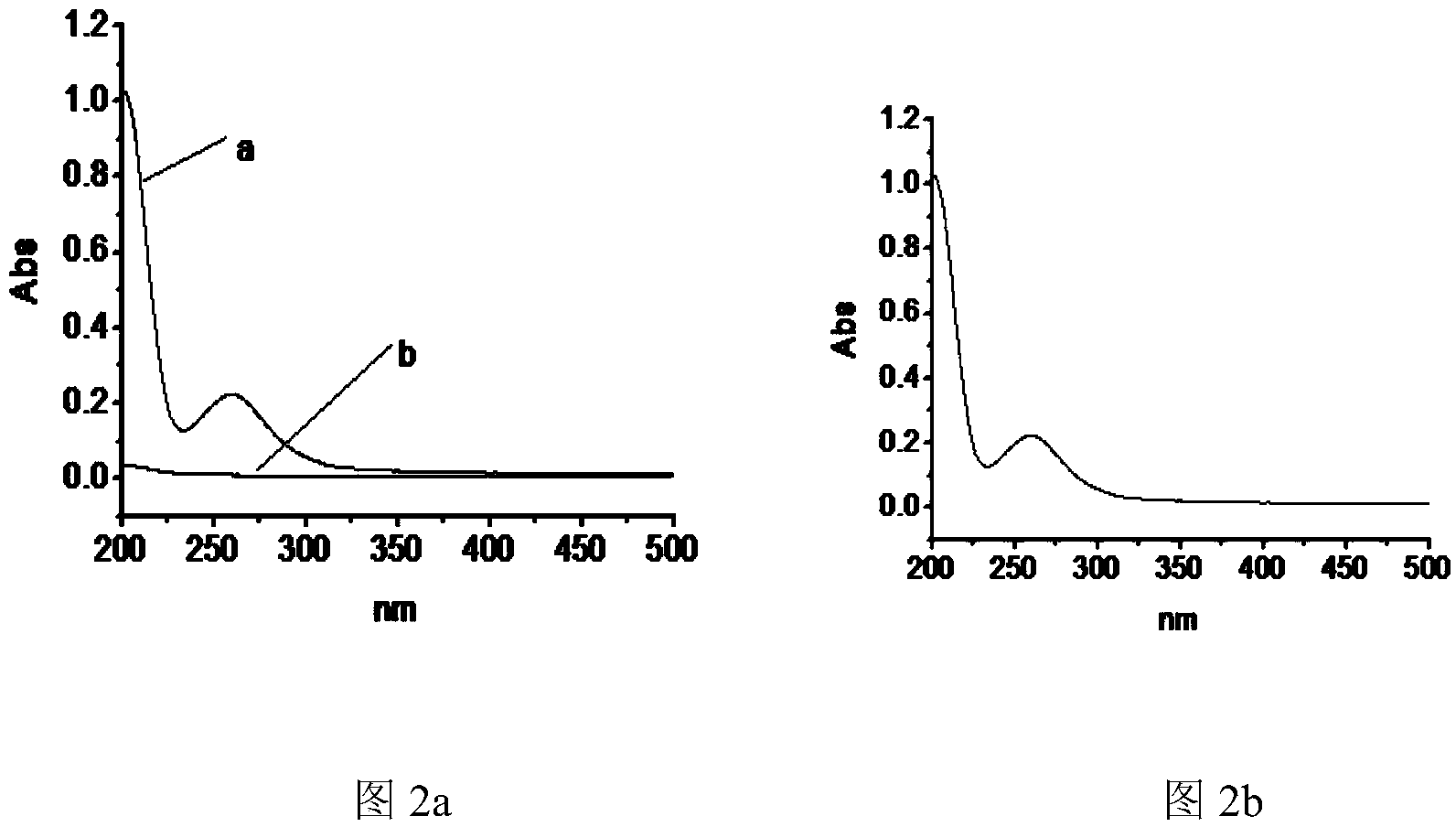
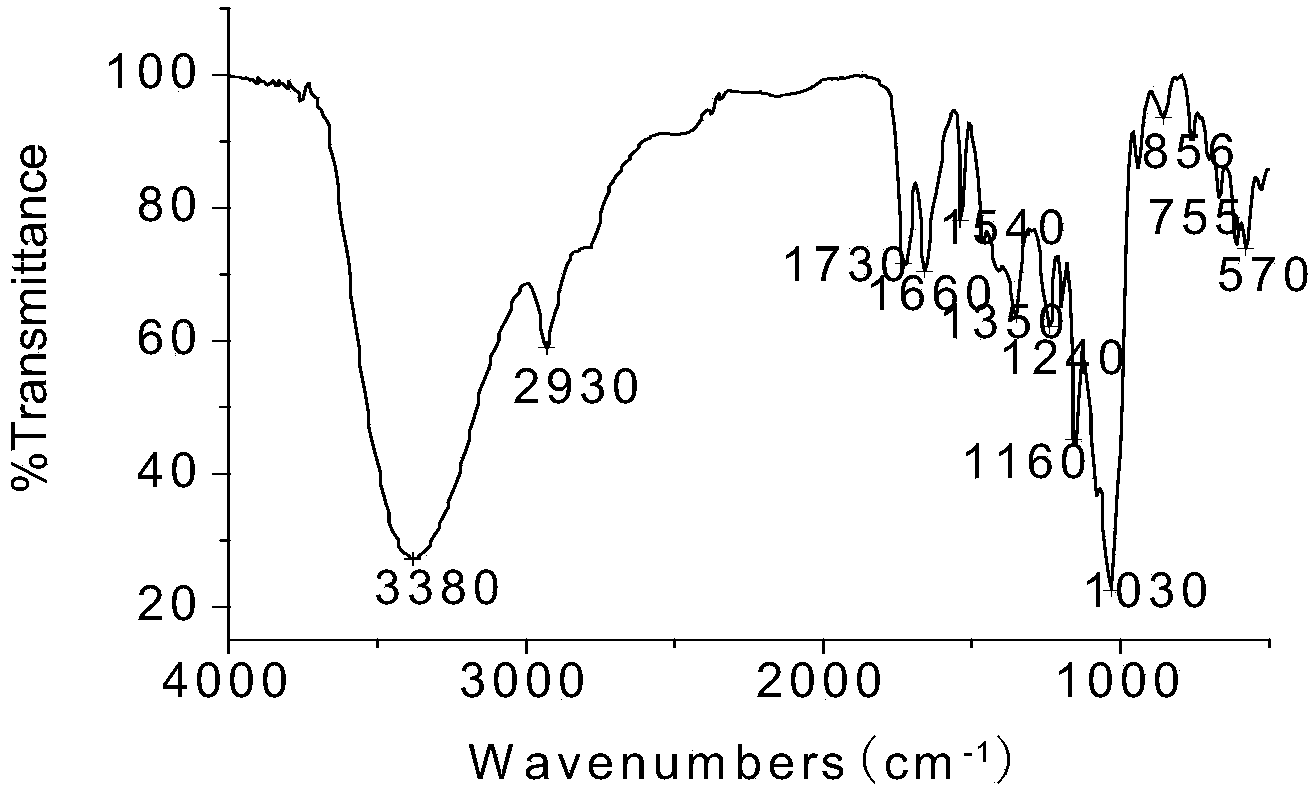
[0080] A new type of chiral separation material, bis-(6-oxo-m-nitrobenzenesulfonyl)-β-cyclodextrin, was used to prepare a HPCE monolithic column for high performance capillary electrophoresis. Using scanning electron microscopy, it was confirmed that there was a dense layer in the capillary column , there is also a large gap.
[0081] 1. Preparation method of high performance capillary electrophoresis column
[0082] The preparation method is to pretreat an empty HPCE capillary column (Yongnian, Hebei, with an inner diameter of 75 μm and a column length of 55 cm) (washing with methanol, sodium hydroxide, and hydrochloric acid in turn to activate the column), and then inject 1 μmol of bis-(6-oxo-methylene Nitrobenzenesulfonyl)-β-cyclodextrin, 15 μmol glycidyl methacrylate, 6 μmol ethylene glycol dimethacrylate, and 30 μmol azobisisobutyronitrile were injected into the tube and filled, and re...
Embodiment 2
[0087] Using the chiral bis-(6-oxo-m-nitrobenzenesulfonyl)-β-cyclodextrin monolithic column prepared in Example 1 to separate chiral drugs cetirizine, mexiletine, ofloxacin, and bubi Caine and propafenone
[0088] 1. Main instruments and reagents
[0089] 1.1 Instrument
[0090] HPCE instrument (Beijing Cailu, CL1020 type); HPCE instrument (USA BECKMAN, MDQ type); pH acidity meter (Shanghai Weiye, ZD-2 type); ultrasonic cleaner (Kunshan Ultrasonic Instrument, KQ2200B type); electronic analytical balance (Shanghai Sunny, FA2004); electromagnetic stirrer (Shanghai Weiye, CJ-1); fused silica capillary (Hebei Yongnian, 50cm×Φ75μm); syringe filter (Tianjin Jinteng, 0.22μm); Shaxing Tablets (Guangzhou Baiyun Mountain); Ofloxacin Reference Substance (National Institute for Drug Control, Content 98.8%); Bupivacaine Injection (Shanghai Hefeng); Bupivacaine Reference Substance (National Institute for Drug Control, Content 98.8%) %); Cetirizine Hydrochloride Tablets (Suzhou Dongrui); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com