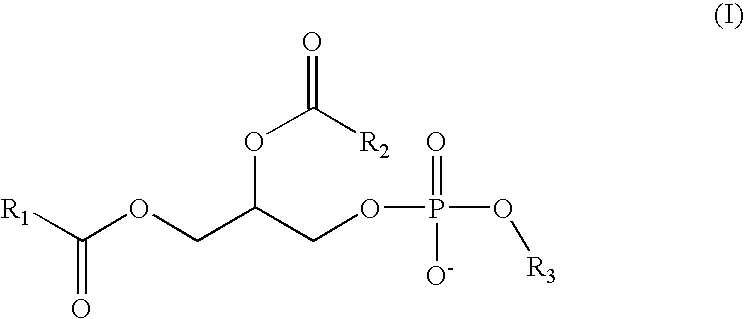
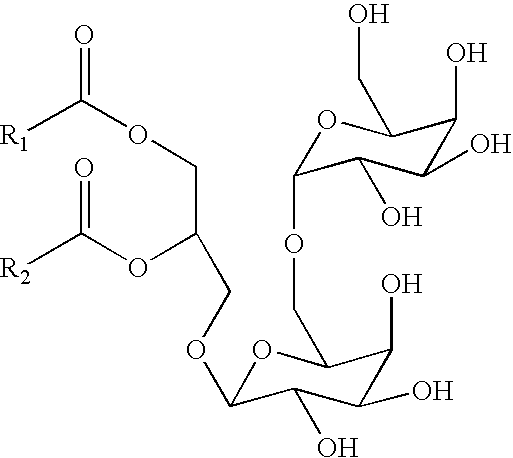
Method and composition for treating rhinitis
a technology for rhinitis and composition, applied in the field of rhinitis treatment, can solve the problems of side effects of sedation and dry mouth, adverse effects on treatment efficacy, etc., and achieve the effect of protecting the nasal mucosa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0054]
TABLE 1Batch formula of the composition of the inventionCetirizine dinitrate*22.2gPhospholipid (from soybean**)70.0gDisodium phosphate dihydrate; Na2HPO4.2H2O21.3gPotassium dihydrogenphosphate; KH2PO411.0g1 M Hydrochloric acid and / or 1 M sodium hydroxideto pH 7.0Water for injectionto 2.0L
*White solid, crystallized from THF / acetonitrile / water 2:1:0.28. Obtained from commercially available cetirizine dihydrochloride via neutralisation of the free base with nitric acid.
**Lipoid S75, Lipoid GmbH, Germany
[0055] General procedure. For weights and volumes reference is made to Table 1. A buffer solution is prepared by dissolving the buffering agents disodium phosphate dihydrate (Na2HPO4.2H2O) and potassium dihydrogen phosphate (KH2PO4) in 1600 ml water (80% of the total batch volume) in a 2000 mL volumetric flask. The weighed amount of active agent is added to the buffer solution and dissolved by stirring with a magnetic stirrer, followed by addition of 100 ml aqueous 1 M sodium hyd...
example 2
[0058]
TABLE 3Batch formula of the composition of the inventionCetirizine dinitrate2.22mgPhospholipid (soybean; Lipoid S75;7.00mgLipoid GmbH, Germany)Citric acid, anhydrous3.84mgSodium hydroxide, solid1.67mgAscorbic acid0.20mgEDTA sodium0.20mgHCl, 1 M and / or NaOH, 1 MTo pH 5.0Water for injectionTo 200mL
[0059] General procedure. For weights and volumes reference is made to Table 3. A buffer solution is prepared by dissolving anhydrous citric acid and solid sodium hydroxide in 160 mL water (80% of the total batch volume) in a 200 mL volumetric flask. The weighed amount of active agent is added and dissolved by stirring with a magnetic stirrer. The phospholipid is separately weighed and added to the cetirizine solution. Stirring is continued until a well dispersed suspension has been formed, the pH of which is adjusted to pH 5.0±0.1 with 1.0 M NaOH and / or 1.0 M HCl. The volume of the preparation is then brought to the final batch volume of 200 mL. The preparation is transferred to a hig...
example 3
[0062]
TABLE 5Composition of the inventionCetirizine dinitrate5.6mgPhospholipid (soybean; Lipoid S75; Lipoid GmbH,35.0mgGermany)Disodium phosphate dihydrate; Na2HPO4.2H2O10.7mgPotassium dihydrogen phosphate; KH2PO45.5mg1 M HCl and / or 1 M NaOHTo pH 7.0Water for injectionTo 1mL
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com