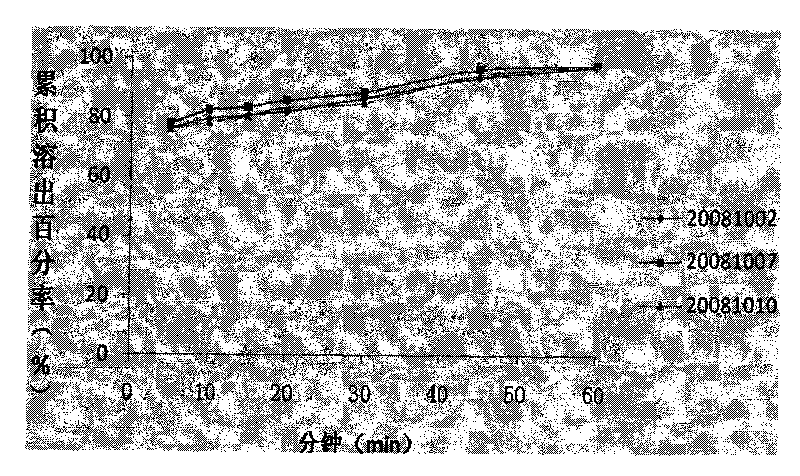
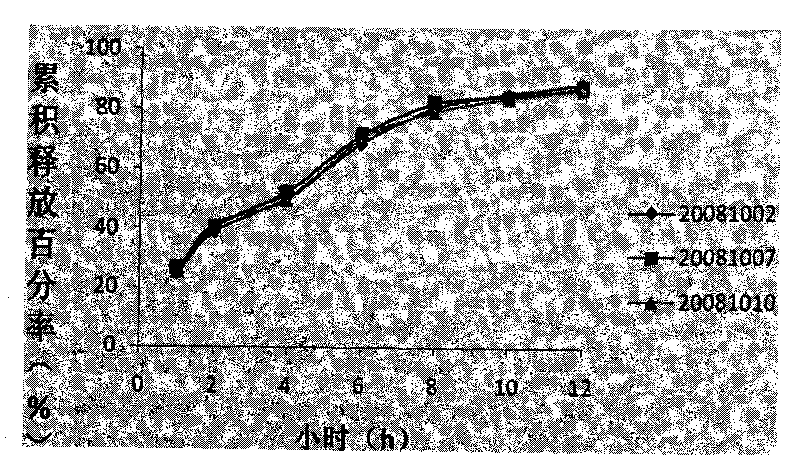
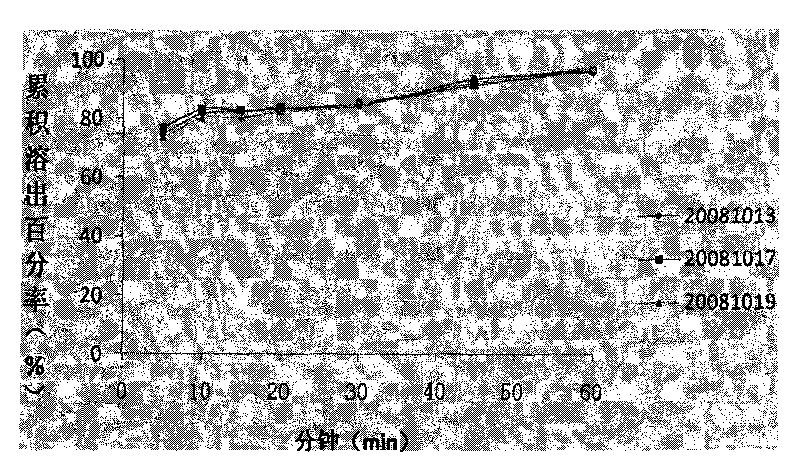
Compound sustained-release tablet of cetirizine and pseudoephedrine and preparation method thereof
A technology for cetirizine and pseudoephedrine, applied in the field of medicine, can solve the problems of difficulty in controlling the sudden release of pseudoephedrine, the influence of the immediate release layer, the appearance of spots on the surface, and the like, so as to reduce the number of times of taking medicines, the tablet weight is small, and the tablet is smooth and beautiful. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Preparation of Pseudoephedrine Hydrochloride Sustained Release Tablet Core
[0053] The prescription is (per 1000 tablets):
[0054] A: Pseudoephedrine hydrochloride 120g
[0055] B: Hypromellose (k100m) 120g
[0056] C: Stearic acid 40g
[0057] D: Magnesium stearate (internal addition) 20g
[0058] E: Magnesium stearate (additional) 20g
[0059] F: Polyvinylpyrrolidone (K30) 25g
[0060] G: 95% ethanol 500g
[0061] Crush materials A to D, pass through a 100-mesh sieve, and mix uniformly to obtain a mixture; completely dissolve F: polyvinylpyrrolidone (K30) in G: 95% ethanol to prepare an adhesive; For granulation, control the drying temperature at 45°C; after the obtained granules are evenly mixed with material E, they are compressed into sustained-release tablet cores on a high-speed tablet press.
[0062] 2, the preparation of cetirizine hydrochloride coating layer
[0063] The prescription is (per 1000 tablets):
[0064] A: Pseudoephedrine Hydrochlorid...
Embodiment 2
[0073] 1. Preparation of Pseudoephedrine Hydrochloride Sustained Release Tablet Core
[0074] The prescription is (per 1000 tablets):
[0075] A: Pseudoephedrine hydrochloride 100g
[0076] B: Hypromellose (k100m) 100g
[0077] C: Magnesium stearate (internal addition) 20g
[0078] D: calcium hydrogen phosphate 10g
[0079] E: Magnesium stearate (additional) 20g
[0080] F: Hypromellose (E4) 20g
[0081] G: 50% ethanol 400g
[0082] Crush materials A to D, pass through a 100-mesh sieve, and mix uniformly to obtain a mixture; completely dissolve F: hypromellose (E4) in G: 50% ethanol to prepare an adhesive; The mixture is granulated, and the drying temperature is controlled at 45°C; after the obtained granules are evenly mixed with material E, they are compressed into sustained-release tablet cores on a high-speed tablet press.
[0083] 2, the preparation of cetirizine hydrochloride coating layer
[0084] The prescription is (per 1000 tablets):
[0085] A: Pseudoephedr...
Embodiment 3
[0094] 1. Preparation of Pseudoephedrine Hydrochloride Sustained Release Tablet Core
[0095] The prescription is (per 1000 tablets):
[0096] A: Pseudoephedrine hydrochloride 240g
[0097]B: Hypromellose (k100m) 150g
[0098] C: Stearic acid 80g
[0099] D: Magnesium stearate (internal addition) 40g
[0100] E: Magnesium stearate (additional) 20g
[0101] F: Polyvinylpyrrolidone (K30) 50g
[0102] G: 95% ethanol 1000g
[0103] Crush materials A to D, pass through a 100-mesh sieve, and mix uniformly to obtain a mixture; completely dissolve F: polyvinylpyrrolidone (K30) in G: 95% ethanol to prepare an adhesive; For granulation, control the drying temperature at 45°C; after the obtained granules are evenly mixed with material E, they are compressed into sustained-release tablet cores on a high-speed tablet press.
[0104] 2. Preparation of levocetirizine hydrochloride coating layer
[0105] The prescription is (per 1000 tablets):
[0106] A: Pseudoephedrine Hydrochlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com