Cetirizine molecularly imprinted polymer monolithic column and preparation method thereof
A cetirizine and molecular imprinting technology, which is applied in the field of preparation of molecularly imprinted monolithic columns, can solve problems such as non-volatile, and achieve the effects of easy operation, simple preparation process and reduced operation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation and separation of cetirizine and its six-membered heterocyclic triazine structural analogs containing nitrogen atoms on a molecularly imprinted monolithic column for cetirizine
[0028] A molecularly imprinted monolithic column with cetirizine as template was synthesized by in situ polymerization, and its retention performance was evaluated by connecting it to high performance liquid chromatography under appropriate chromatographic conditions. Synthetic reaction conditions and processing method are as follows:
[0029] Preparation of cetirizine molecularly imprinted monolithic column based on deep eutectic solvent by in situ polymerization:
[0030] a. According to the mass number of cetirizine imprinted monolithic column synthesized in a stainless steel column, accurately weigh the template molecule as cetirizine 1.56%, the functional monomer as 4-VP (4-vinylpyridine) 3.43%, The crosslinking agent is 23.4% ethylene glycol dimethacrylate, and the porogen is...
Embodiment 2
[0034] Embodiment 2 (control)
[0035] Separation of Cetirizine and Its Structural Analogues on a Blank Monolithic Column
[0036] In order to investigate the retention of cetirizine and its structural analogues on non-imprinted columns, a blank column without template cetirizine was synthesized as a control. The specific operation steps are as follows:
[0037] A non-imprinted blank column was synthesized with the same method and experimental conditions as in Example 1, except that the template molecule cetirizine was not added. After polymerization is complete, flush with acetonitrile to remove residual porogen and unreacted reagents from the monolith.
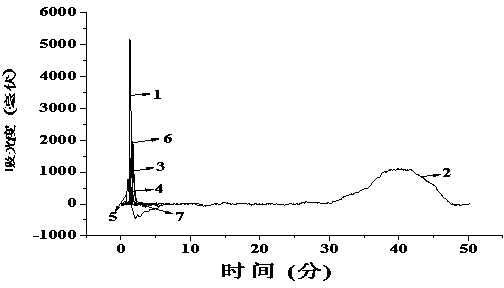
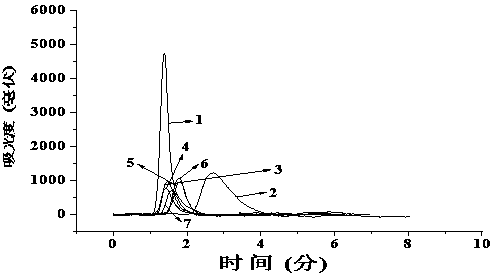
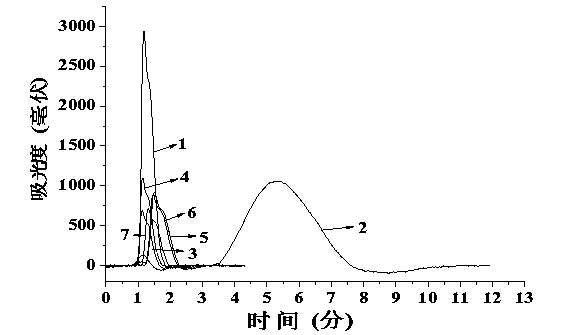
[0038] The chromatographic evaluation is the same as the investigation of the imprinted column in Example 1, that is, under the same mobile phase conditions, by measuring the retention time t of cetirizine and its six-membered heterocyclic triazine analogs containing nitrogen atoms R and retention time t of acetone 0 , t...
Embodiment 3
[0042] Preparation of cetirizine molecularly imprinted monolithic column by in situ polymerization:
[0043] a. According to the mass number of cetirizine imprinted monolithic column synthesized in a stainless steel column, weigh 1.56% of cetirizine, 3.43% of 4-VP (4-vinylpyridine) as the functional monomer, and 3.43% of the crosslinking agent as Ethylene glycol dimethacrylate 24.3%, porogen is a deep eutectic solvent composed of ethylene glycol dissolved in choline chloride 44.1%, DMF (dimethylformamide) 4.28%, ionic liquid (1-butyl base-3-methylimidazolium tetrafluoroborate) 20.4%, then add 0.99% cobalt acetate and 0.94% azobisisobutyronitrile as the initiator to mix, put it into the ultrasonic machine, and ultrasonicate for 20 minutes to make it uniform. Clarify, then transfer to a clean stainless steel column (1004.6 mm I.D.), ultrasonically degas for 15 minutes, seal both ends of the stainless steel column, and react in a constant temperature water bath at a temperature o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com