Tablet containing olmesartan medoxomil and amlodipine and preparation method of tablet
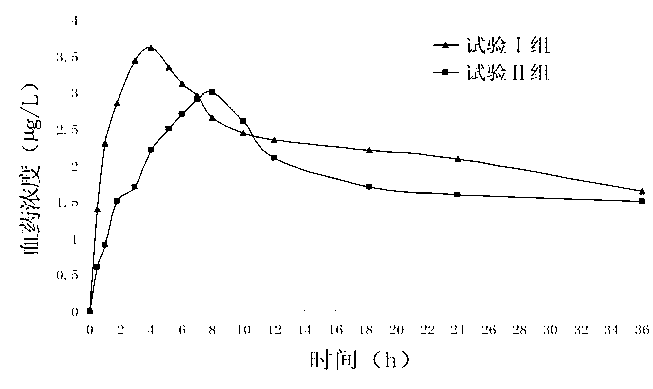
A technology of olmesartan medoxomil and amlodipine besylate, which is applied in the field of tablets containing olmesartan medoxomil and amlodipine and its preparation, can solve the problem of low overall level of blood drug concentration, limited complementary effect, and poor blood drug concentration. problems such as low concentration, to achieve the effect of reducing the incidence of adverse drug reactions, increasing the dissolution rate, and reducing the incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 50g of amlodipine besylate in 2.2L of dichloromethane, add 436g of hydroxypropyl methylcellulose, stir to dissolve, and rotate to evaporate below 45°C. After dichloromethane is evaporated, quickly pour it into a petri dish and lay it flat. Place it in the freezer layer of the refrigerator for about 5 hours, then freeze-dry it for 20 hours, wait for embrittlement, take it out and pulverize it and pass it through an 80-mesh sieve to obtain a solid dispersion of amlodipine besylate.
Embodiment 2
[0026] Dissolve 50g of amlodipine besylate in 1.7L of methanol, add 520g of hydroxypropyl methylcellulose, stir to dissolve, and rotate to evaporate below 45°C. After evaporating methanol, quickly pour it into a petri dish and spread it in a freezer layer. Stand for about 6 hours, then freeze-dry for 24 hours, wait for embrittlement, take out and pulverize and pass through an 80-mesh sieve to obtain amlodipine besylate solid dispersion. Example 3
Embodiment 3
[0027]
[0028] Preparation process: Weigh and mix the amlodipine besylate solid dispersion prepared in Example 1, olmesartan medoxomil, mannitol, lactose, sodium carboxymethyl starch and magnesium stearate, and press into 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com