Valsartan amlodipine tablet composition and preparation method
A technology of amlodipine and amlodipine besylate, which can be applied in directions such as drug combinations, pharmaceutical formulations, and active ingredients of heterocyclic compounds, can solve problems such as low bioavailability, large differences in tablet weight, and poor particle fluidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
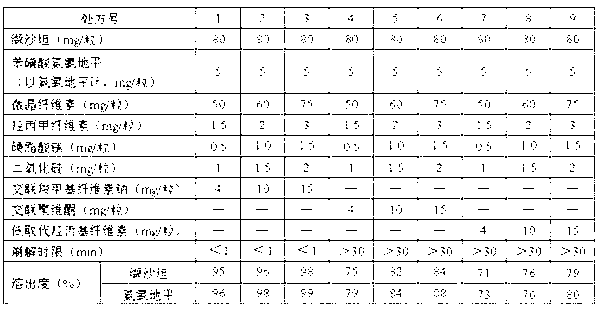
[0047] 1. Valsartan and amlodipine tablets 1000 tablets, the specifications are 80mg of valsartan and 5mg of amlodipine besylate per tablet, the formula composition is as follows:
[0048] Valsartan 80g,
[0049] Amlodipine besylate (calculated as amlodipine) 5g,
[0050] Microcrystalline cellulose 50g,
[0051] Croscarmellose Sodium 4g,
[0052] Hydroxypropylmethylcellulose E15 1.5g,
[0053] Magnesium stearate 0.5g,
[0054] Silicon dioxide 1g.
[0055] 2, adopt the following method to prepare valsartan amlodipine film-coated tablet:
[0056] 1) Pass valsartan, amlodipine besylate, and microcrystalline cellulose through an 80-mesh sieve, and set aside;
[0057] 2) Prepare a 2% solution of hydroxypropyl methylcellulose with purified water for later use;
[0058] 3) Weigh the above spare valsartan, amlodipine besylate, microcrystalline cellulose and 1 / 2 croscarmellose sodium according to the prescription amount, mix them uniformly by equal addition method, and then add ...
Embodiment 2
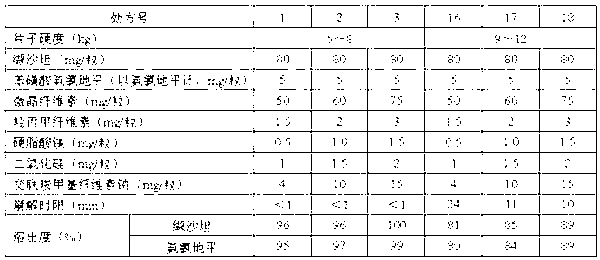
[0064] 1. Valsartan and amlodipine tablets 1000 tablets, the specifications are 80mg of valsartan and 5mg of amlodipine besylate per tablet, the formula composition is as follows:
[0065] Valsartan 80g,
[0066] Amlodipine besylate (calculated as amlodipine) 5g,
[0067] Microcrystalline Cellulose 75g,
[0068] Croscarmellose Sodium 15g,
[0069] Hydroxypropyl Methyl Cellulose E15 3g,
[0070] Magnesium Stearate 1.5g,
[0071] Silica 2g.
[0072] 2, adopt the following method to prepare valsartan amlodipine film-coated tablet:
[0073] 1) Pass valsartan, amlodipine besylate, and microcrystalline cellulose through a 100-mesh sieve respectively, and set aside;
[0074] 2) Prepare a 5% solution of hydroxypropyl methylcellulose with purified water for later use;
[0075] 3) Weigh the above spare valsartan, amlodipine besylate, microcrystalline cellulose and 1 / 2 croscarmellose sodium according to the prescription amount, mix them uniformly by equal addition method, and then a...
Embodiment 3
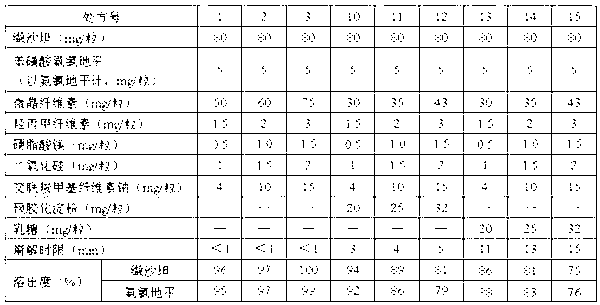
[0081] 1. Valsartan and amlodipine tablets 1000 tablets, the specifications are 80mg of valsartan and 5mg of amlodipine besylate per tablet, the formula composition is as follows:
[0082] Valsartan 80g,
[0083] Amlodipine besylate (calculated as amlodipine) 5g,
[0084] Microcrystalline Cellulose 65g,
[0085] Croscarmellose Sodium 13.6g,
[0086] Hydroxypropylmethylcellulose 15CP 2.3g,
[0087] Magnesium Stearate 0.8g,
[0088] Silica 1.5g.
[0089] 2, adopt the following method to prepare valsartan amlodipine film-coated tablet:
[0090] 1) Pass valsartan, amlodipine besylate, and microcrystalline cellulose through a 90-mesh sieve respectively, and set aside;
[0091] 2) Prepare a 5% solution of hydroxypropyl methylcellulose with purified water for later use;
[0092] 3) Weigh the above spare valsartan, amlodipine besylate, microcrystalline cellulose and 1 / 2 croscarmellose sodium according to the prescription amount, mix them uniformly by equal addition method, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com