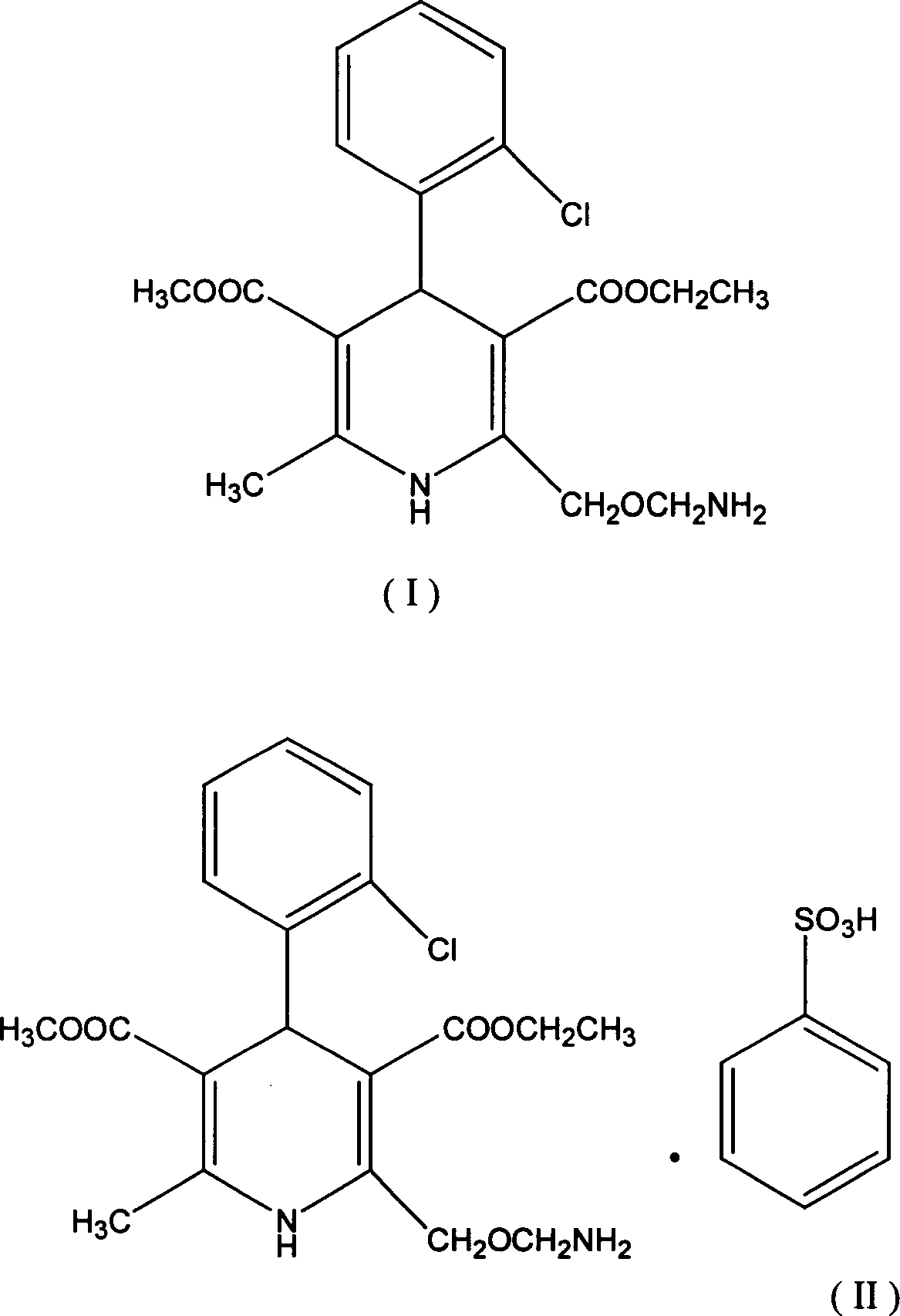
Method for preparing amlodipine benzenesulfonate
A kind of technology of amlodipine besylate and amlodipine, which is applied in the field of preparation of amlodipine besylate, can solve the problems of increasing reaction steps, increasing costs, unfavorable product quality control, etc., and achieves simple operation process and high yield High, easy-to-control product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Mix 99% isopropyl acetate and 99% isopropanol in a volume ratio of 5:1 to form a mixed solvent.
[0017] Dissolve 10g of amlodipine base (24.46mmol) in 80ml of the aforementioned mixed solvent, maintain the temperature at 0-5°C, and slowly add dropwise 4g (25.3mmol) of anhydrous benzenesulfonic acid dissolved in 40ml of the aforementioned mixed solvent. Colorless crystals were precipitated during the dropwise addition, and the reaction was continued for 2.5 hours after the addition was completed. Suction filtration, wash the filter cake with 20ml of mixed solvent, and vacuum-dry at below 50°C for 6 hours to obtain 13.2g of amlodipine besylate with a yield of 95.2%. All indicators of the product meet the existing drug standards.
Embodiment 2
[0019] Mix 99% isopropyl acetate and 99% isopropanol according to the volume ratio of 1:1 to form a mixed solvent.
[0020] Dissolve 10g of amlodipine base (24.46mmol) in 80ml of the aforementioned mixed solvent, maintain the temperature at 0-5°C, and slowly add dropwise 4g (25.3mmol) of anhydrous benzenesulfonic acid dissolved in 40ml of the aforementioned mixed solvent. Colorless crystals were precipitated during the dropwise addition, and the reaction was continued for 2.5 hours after the addition was completed. Suction filtration, wash the filter cake with 20ml of mixed solvent, and vacuum-dry at below 50°C for 6 hours to obtain 12.5g of amlodipine besylate with a yield of 90.1%.
Embodiment 3
[0022] Mix 99% isopropyl acetate and absolute ethanol in a volume ratio of 1:1 to form a mixed solvent.
[0023] Dissolve 10g of amlodipine base (24.46mmol) in 80ml of the aforementioned mixed solvent, maintain the temperature at 0-5°C, and slowly add dropwise 4g (25.3mmol) of anhydrous benzenesulfonic acid dissolved in 40ml of the aforementioned mixed solvent. Colorless crystals were precipitated during the dropwise addition, and the reaction was continued for 2.5 hours after the addition was completed. Suction filtration, wash the filter cake with 20 ml of mixed solvent, and vacuum-dry at below 50° C. for 6 hours to obtain amlodipine besylate 12.0, with a yield of 86.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com