Phenylsulfonic acid amido chloro diping dispersion tablet and its preparation method
A technology of amlodipine besylate and amlodipine besylate, which is applied in the field of pharmaceuticals, can solve the problems that amlodipine besylate dispersible tablets have not yet been listed on the market, achieve good clinical application prospects, effectively reduce blood pressure, and take convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
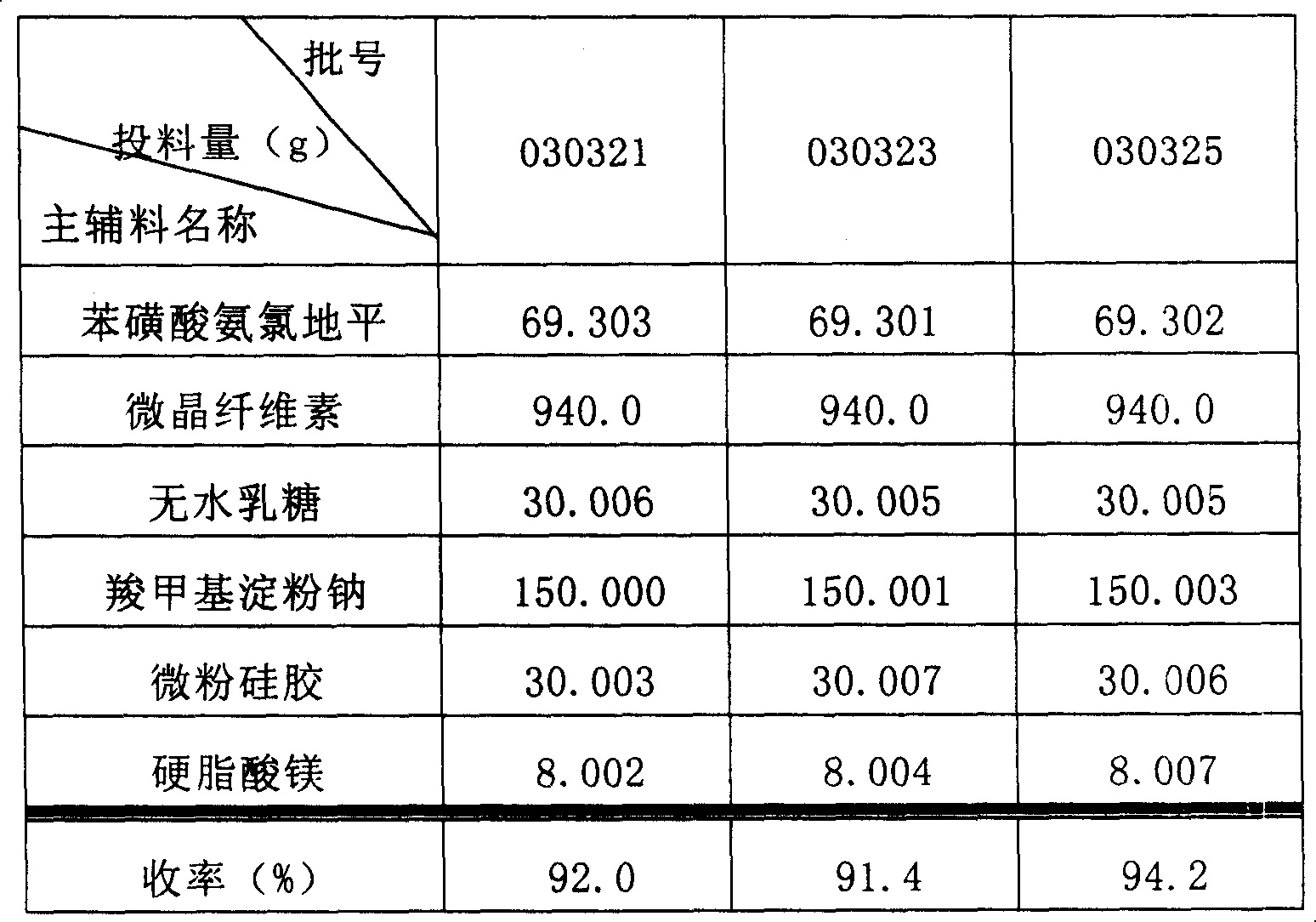
[0034] Embodiment 1: raw material formula:
[0035] Amlodipine besylate 6.93g
[0036] Microcrystalline Cellulose 100.00g
[0037] Anhydrous lactose 30.00g
[0038] Sodium carboxymethyl starch 15.00g
[0039] Micronized silica gel 3.00g
[0040] Magnesium stearate 0.80g
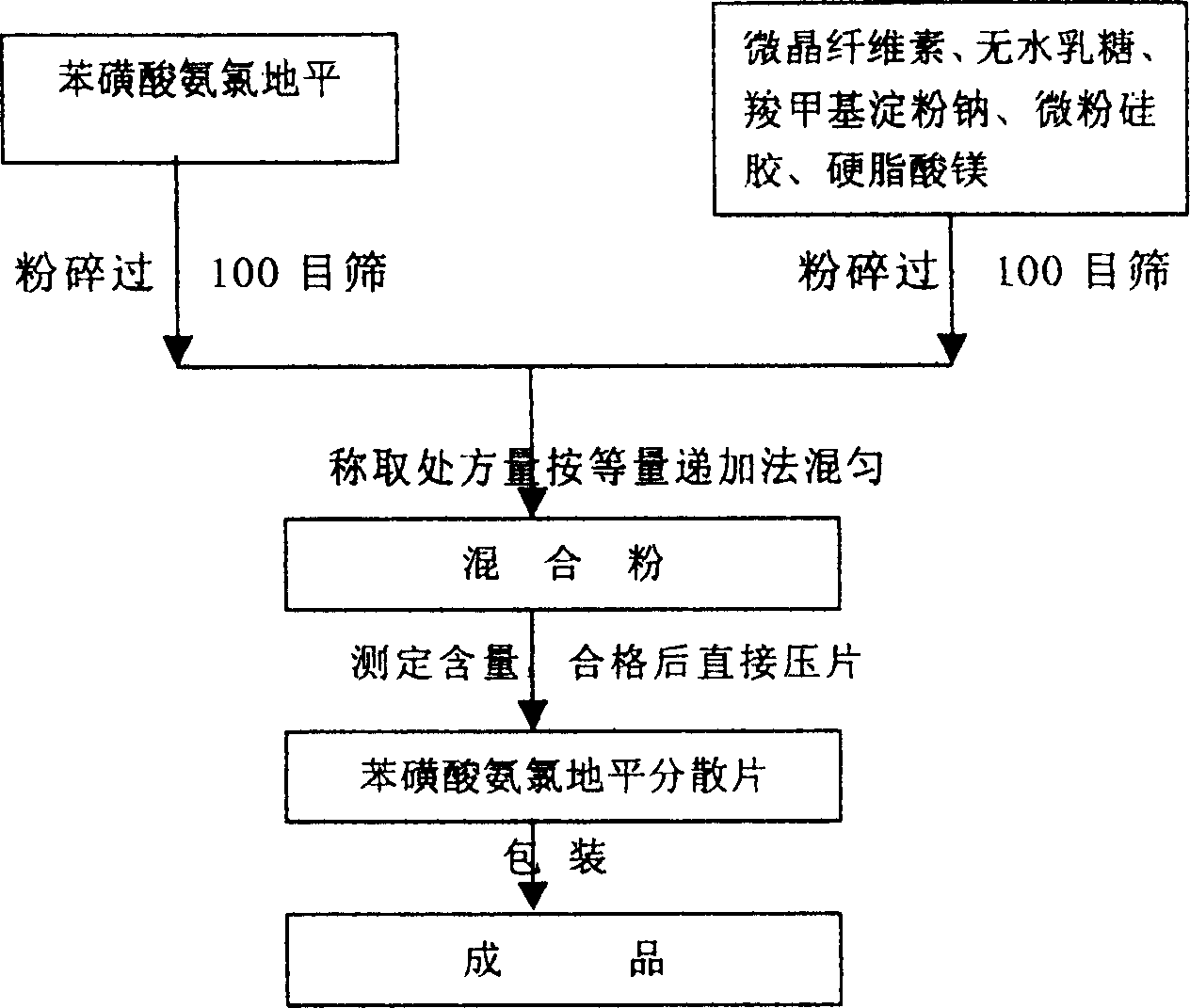
[0041] Preparation method: pulverize amlodipine besylate, pass through a 100-mesh sieve after drying, and set aside; separately pulverize auxiliary materials microcrystalline cellulose, anhydrous lactose, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate, then dry, Pass through a 100-mesh sieve, and set aside; mix the above-mentioned main ingredients and auxiliary materials uniformly by the method of equal addition, measure the content, find out the theoretical tablet weight after passing the test, directly compress the tablet according to the theoretical tablet weight, and pack it to obtain the product.
Embodiment 2
[0042] Embodiment 2: raw material formula:
[0043] Amlodipine Besylate 6g
[0044] Microcrystalline Cellulose 70g
[0045] Anhydrous lactose 20g
[0046]Sodium carboxymethyl starch 14g
[0047] Micronized silica gel 2g
[0048] Magnesium stearate 0.85g
[0049] The preparation method is prepared according to the method of Example 1.
Embodiment 3
[0050] Embodiment 3: raw material formula:
[0051] Amlodipine Besylate 7g
[0052] Microcrystalline Cellulose 80g
[0054] Sodium carboxymethyl starch 16g
[0055] Micronized silica gel 4g
[0056] Magnesium Stearate 1.0g
[0057] The preparation method is prepared according to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com