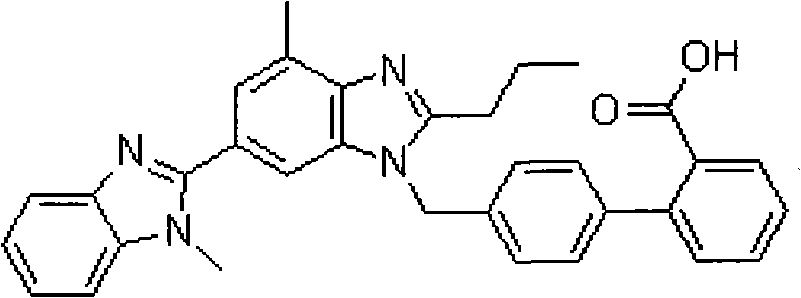
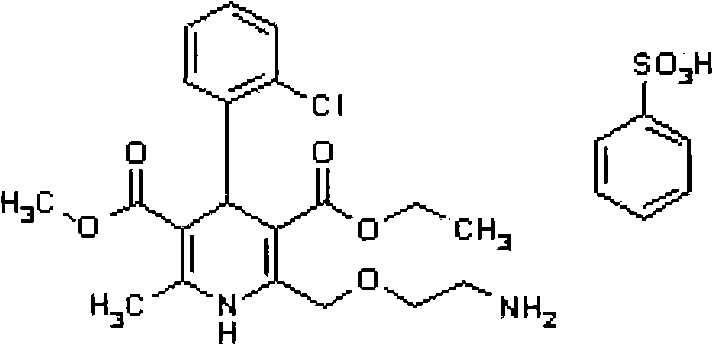
Preparation method for coating tablets containing telmisartan and amlodipine
A technology of telmisartan and amlodipine is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, sugar-coated pills, etc., and can solve problems such as instability of amlodipine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
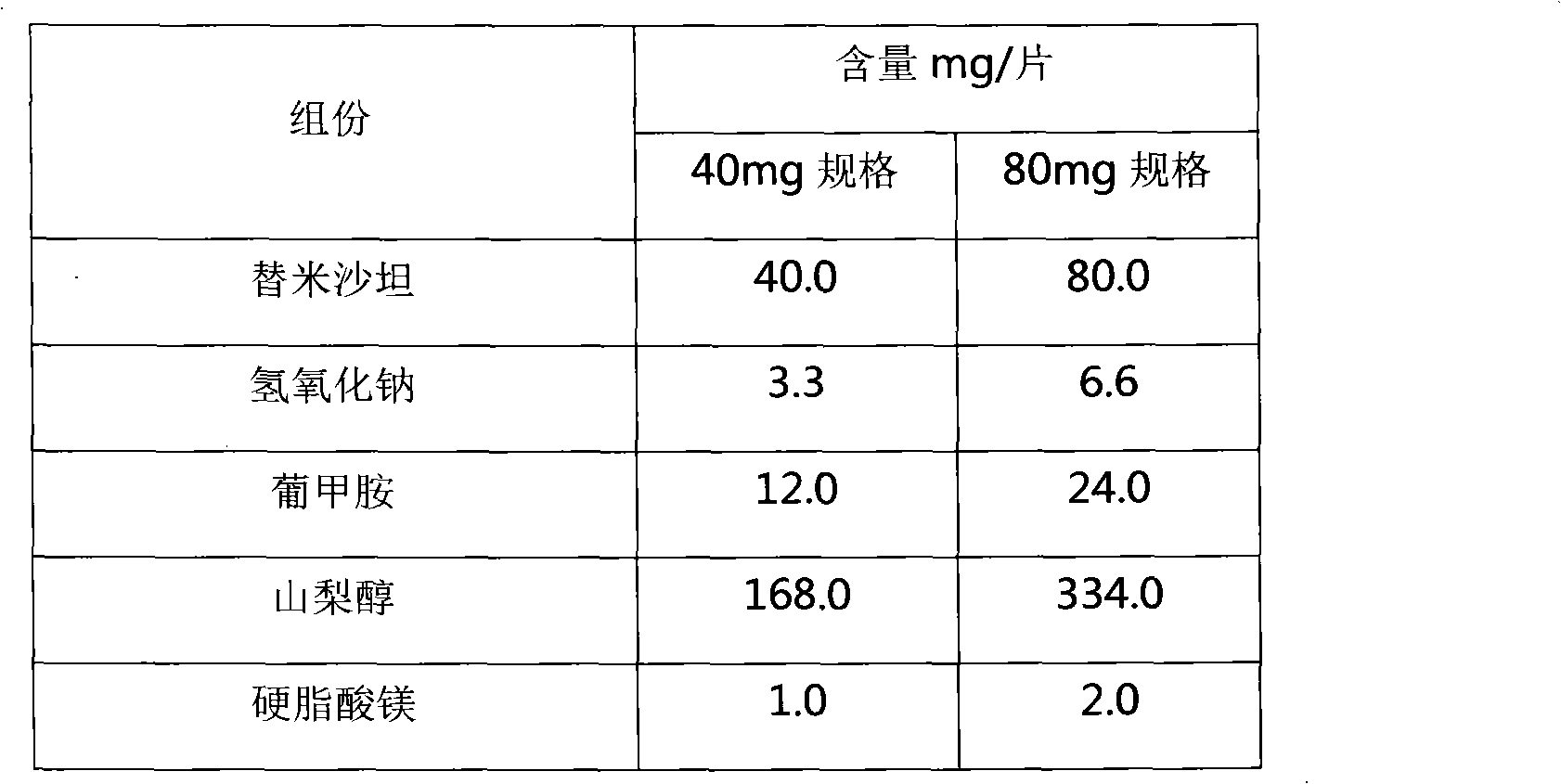
[0035] Tablet core prescription: divided into two specifications of telmisartan 40mg and 80mg, the prescription of the two specifications in the tablet core increases proportionally.
[0036]
[0037] Preparation method of tablet core:
[0038] 1. Weigh telmisartan, sodium hydroxide and meglumine according to the prescription, add water to dissolve it, spray dry, and collect the spray dried powder. Determine the content.
[0039] 2. According to the prescription composition and content determination result, convert the dosage of sorbitol and magnesium stearate and add to the spray-dried powder. Mix well and compress.
[0040] Coating liquid prescription (1000ml coating liquid dosage)
[0041] Component
Weight (g / 1000ml 50% ethanol)
Hydroxypropyl methylcellulose (viscosity 5 centipoise)
10
Polyethylene glycol 1500 (average molecular weight 1500)
5
Tween 80
4
10
Titanium dioxide
8
55
50% ethanol (volume ratio)
1000ml
[0042] Prepara...
Embodiment 2
[0052] Tablet core prescription: the same as in Example 1.
[0053]
[0054] Preparation method of tablet core: same as in Example 1.
[0055] 1. Weigh telmisartan, sodium hydroxide and meglumine according to the prescription, add water to dissolve it, spray dry, and collect the spray dried powder. Determine the content.
[0056] 2. According to the prescription composition and content determination result, convert the dosage of sorbitol and magnesium stearate and add to the spray-dried powder. Mix well and compress.
[0057] Coating liquid prescription (1000ml coating liquid dosage)
[0058] Coating liquid for isolation layer:
[0059] Component
Weight (g / 1000ml 50% ethanol)
Hydroxypropyl methylcellulose (viscosity 5 centipoise)
10
Polyethylene glycol 1500 (average molecular weight 1500)
5
10
Tween 80
4
Titanium dioxide
8
50% ethanol (weight ratio)
1000ml
[0060] Preparation method:
[0061] 1. Weigh hydroxypropyl methyl cellulose and polyethylene...
Embodiment 3
[0074] Tablet core prescription:
[0075]
[0076] Preparation method of tablet core:
[0077] 1. Weigh telmisartan, sodium hydroxide and meglumine according to the prescription, add water to dissolve it, spray dry, and collect the spray dried powder. Determine the content.
[0078] 2. According to the prescription composition and content determination results, convert the amount of sorbitol, croscarmellose sodium, talc and magnesium stearate, and add them to the spray-dried powder. Mix well and compress.
[0079] In the present invention, a disintegrant is added, taking croscarmellose sodium as an example, but does not restrict the use of other pharmaceutically acceptable disintegrants to any extent. The disintegrant can be cross-linked carboxymethyl cellulose Sodium carboxymethyl starch, sodium carboxymethyl starch, sodium carboxymethyl starch, polyvinylpyrrolidone×L-10, polyvinylpyrrolidone XL, microcrystalline cellulose, dry starch, etc. The definition of disintegrant is the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com