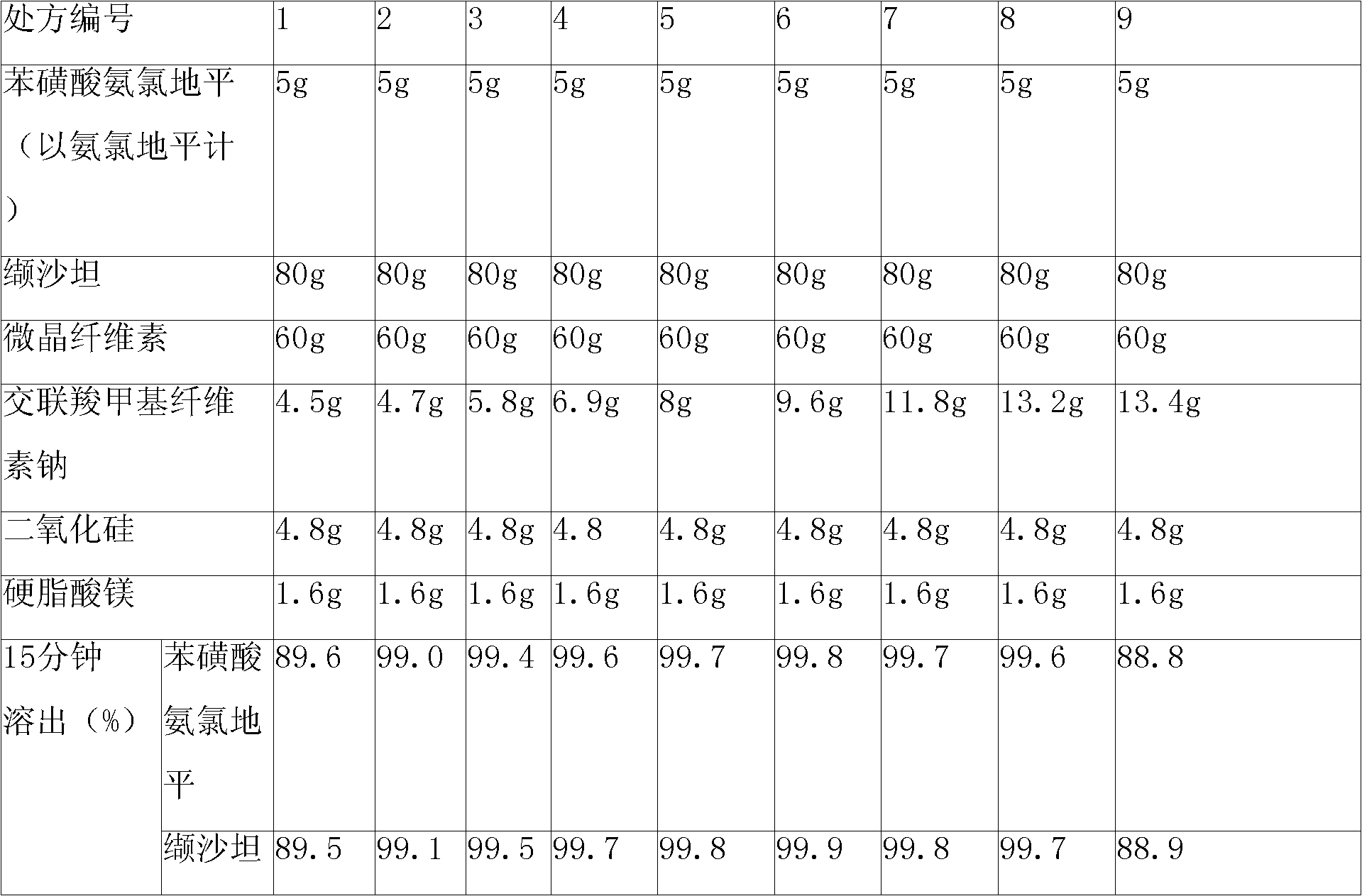Pharmaceutical composition containing Amlodipine besilate and valsartan and preparation method thereof
The technology of amlodipine besylate and amlodipine besylate is applied in directions such as drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve the problems of slow disintegration and dissolution, expensive and complicated equipment, and large dosage And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] 1. Prescription
[0100] Tablet prescription:
[0101] Amlodipine besylate (corresponding to 5g amlodipine free base) 6.94g
[0102] Valsartan 80g
[0103] Microcrystalline Cellulose 60g
[0104] Croscarmellose Sodium 8g
[0105] Silica 4.8g
[0106] Magnesium Stearate 1.6g
[0107] Makes 1000 pieces
[0108] Coating Solution Prescription:
[0109] Opadry II 4.8g
[0110] Purified water 24g
[0111] 28.8g / 1000 tablets
[0112] 2. Preparation process
[0113] (1) Pass the valsartan raw material and the amlodipine besylate raw material through an 80-mesh sieve respectively.
[0114] (2) Dry the microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate at 80°C for 2 hours respectively, and pass through a 60-mesh sieve.
[0115] (3) Take valsartan, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate according to the prescription quantity, and fully mix to obtain a mixed p...
Embodiment 2
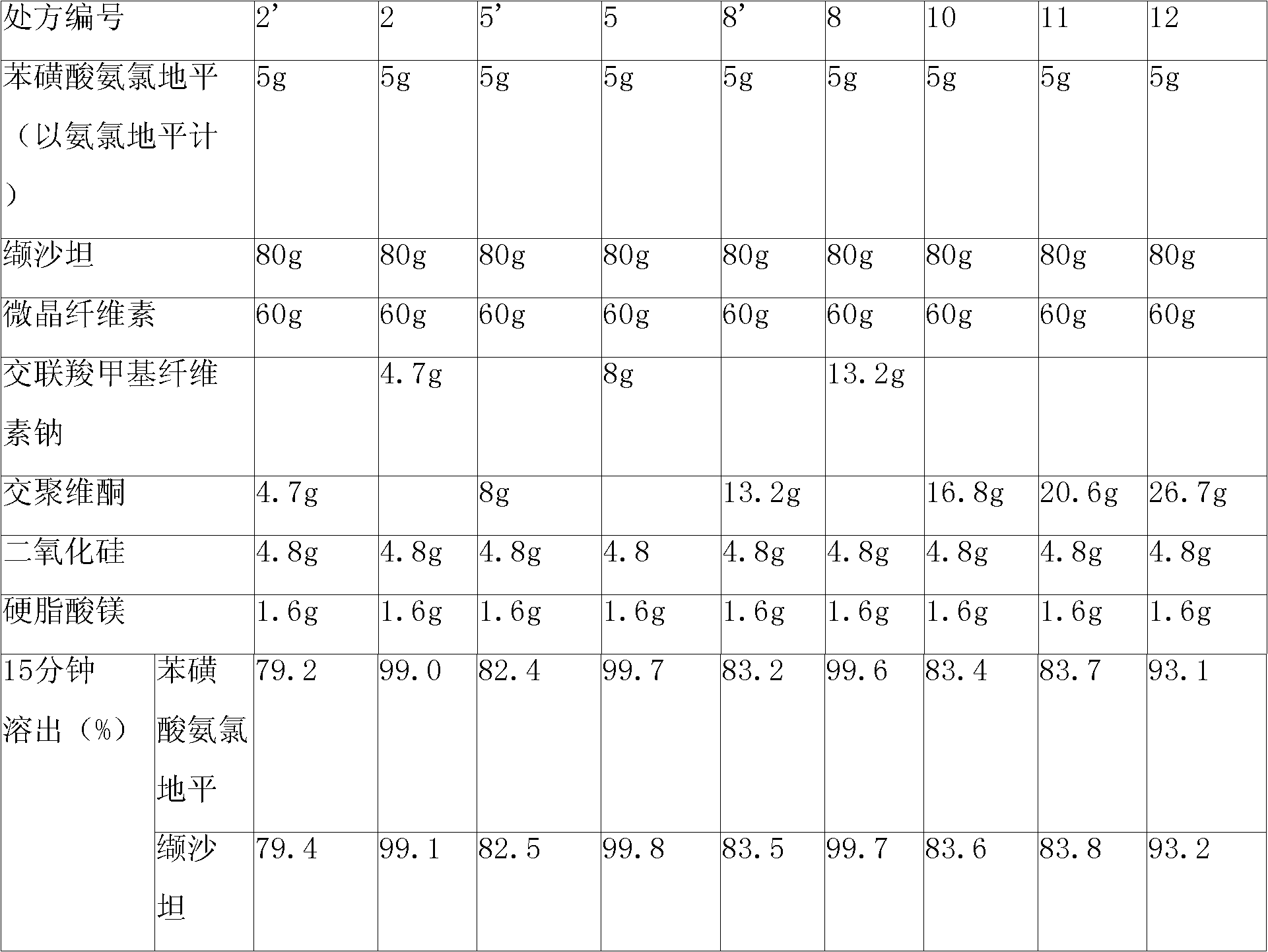
[0121] 1. Prescription
[0122] Tablet prescription:
[0123] Amlodipine besylate (corresponding to 5g amlodipine free base) 6.94g
[0124] Valsartan 80g
[0125] Microcrystalline Cellulose 60g
[0126] Croscarmellose Sodium 4.7g
[0127] Silica 4.8g
[0128] Magnesium Stearate 1.6g
[0129] Makes 1000 pieces
[0130] Coating Solution Prescription:
[0131] Opadry II 4.8g
[0132] Purified water 24g
[0133] 28.8g / 1000 tablets
[0134] 2. Preparation process
[0135] (1) Pass the valsartan raw material and the amlodipine besylate raw material through a 100-mesh sieve respectively.
[0136] (2) Dry the microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate at 60°C for 4 hours, respectively, and pass through a 70-mesh sieve.
[0137] (3) Take valsartan, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate according to the prescription quantity, and fully mix to obtain a mixed powd...
Embodiment 3
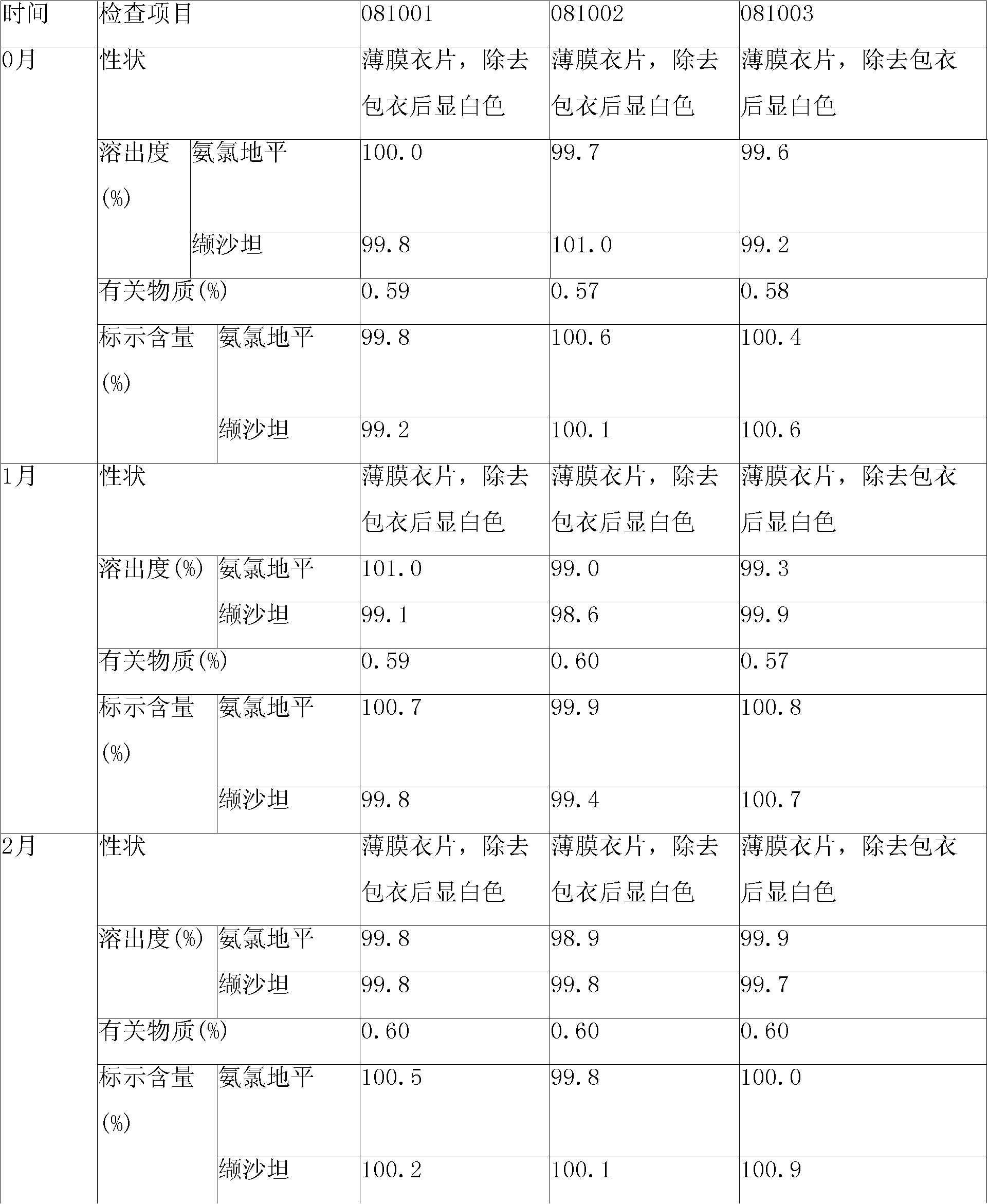
[0143] 1. Prescription
[0144] Tablet prescription:
[0145] Amlodipine besylate (corresponding to 5g amlodipine free base) 6.94g
[0146] Valsartan 80g
[0147] Microcrystalline Cellulose 60g
[0148] Croscarmellose Sodium 13.2g
[0149] Silica 4.8g
[0150] Magnesium Stearate 1.6g
[0151] Makes 1000 pieces
[0152] Coating Solution Prescription:
[0153] Opadry II 4.8g
[0154] Purified water 24g
[0155] 28.8g / 1000 tablets
[0156] 2. Preparation process
[0157] (1) Pass the valsartan raw material and the amlodipine besylate raw material through a 90-mesh sieve respectively.
[0158] (2) Microcrystalline cellulose, croscarmellose sodium, silicon dioxide, and magnesium stearate were baked at 70°C for 3 hours, respectively, and passed through an 80-mesh sieve.
[0159] (3) Take valsartan, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate according to the prescription quantity, and fully mix to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com