Acellular matrix hydrogel as well as preparation method and application thereof
A technology of acellular matrix and hydrogel, which is applied in the fields of pharmaceutical formulations, medical preparations of non-active ingredients, medical science, etc., can solve the problems of poor biological tissue compatibility, species differences, complex extraction process, etc., and achieve sufficient source , Reduce gene mutations, improve survival efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation method of acellular matrix hydrogel
[0041] The embodiment of the present application takes the umbilical cord as the main treatment object, and the preparation method of the present application is also applicable to tissues from other sources. The preparation method of the acellular matrix hydrogel comprises the following steps:
[0042] (1) Obtain the organization block;
[0043] After fresh umbilical cord tissue is obtained, it needs to be frozen at -20°C for transport to the laboratory. Specifically, liquid nitrogen can be used for quick freezing. In the next step, the tissue block needs to be tested for viruses, bacteria, and mycoplasma to ensure that the subsequent acellular matrix hydrogel is free of microbial contamination. After confirming that the tissue block meets the requirements, rinse the superficial blood with deionized water and remove the artery and vein of the umbilical cord for later use.
[0044] (2) Decellularization of tissue block...
Embodiment 2
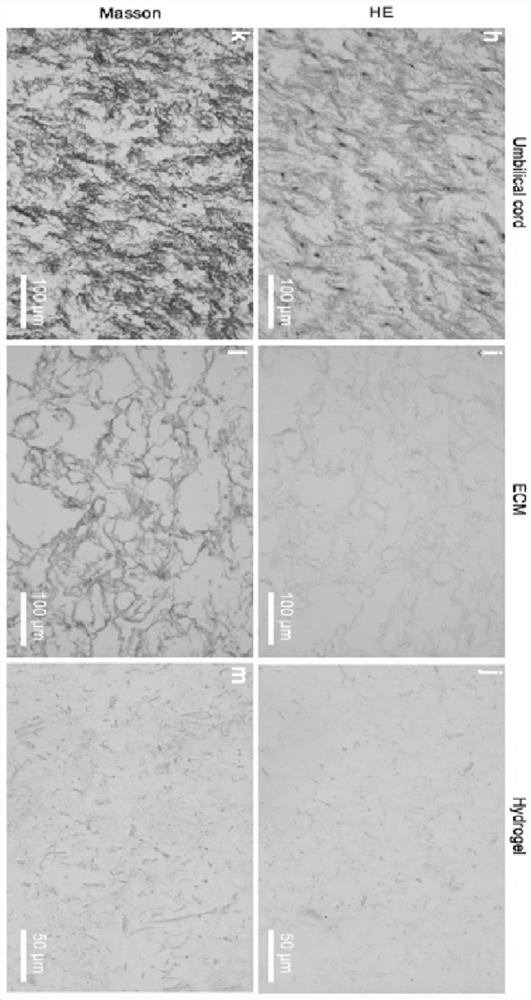
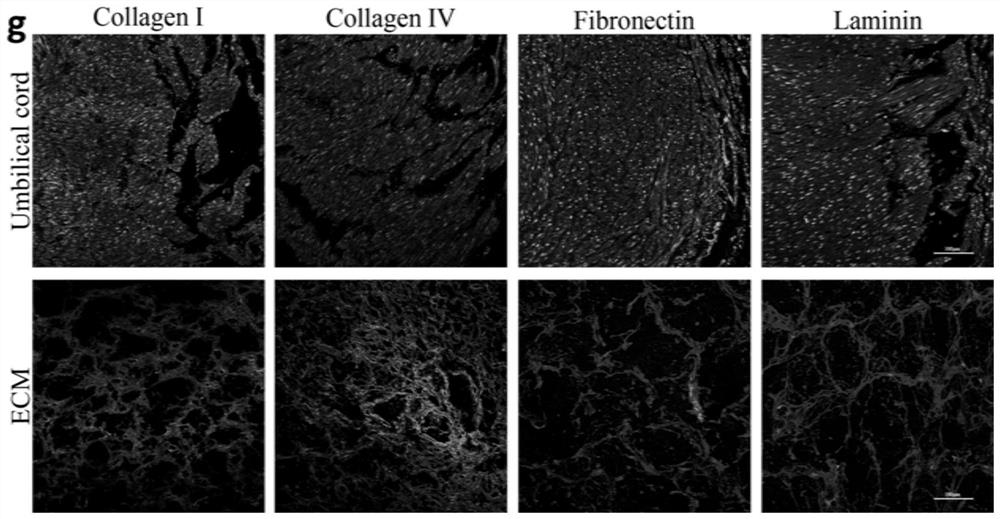
[0067] Standardized identification of acellular matrix hydrogels
[0068] Acellular matrix hydrogels need to meet the specified physical and chemical indicators before they can be applied clinically. Specifically, the standardized identification test items and results of the acellular matrix hydrogel of the present application include:
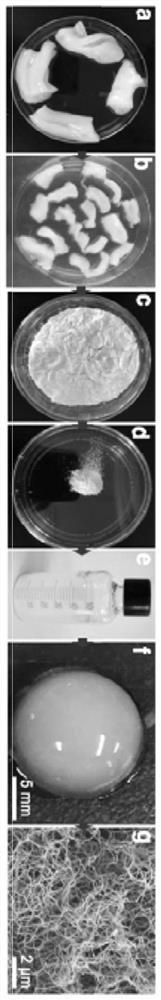
[0069] A. Gelling experiment: under the condition of 37 ℃, the pre-gel was balanced and left at room temperature for 10 minutes to form a hydrogel (see figure 1 f), the hydrogel is a white translucent three-dimensional gel with strong plasticity.
[0070] B. Yield: 3 batches of 10 umbilical cord decellularized scaffolds have a total weight of 16.6g after freeze-drying, and an average of 1.66g of freeze-dried powder can be obtained from each umbilical cord after grinding, which can be made into 160ml pregel.
[0071] C. Scanning electron microscopy: The sample is in the form of agglomerates, and the ultrastructure can be seen under the scanni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com