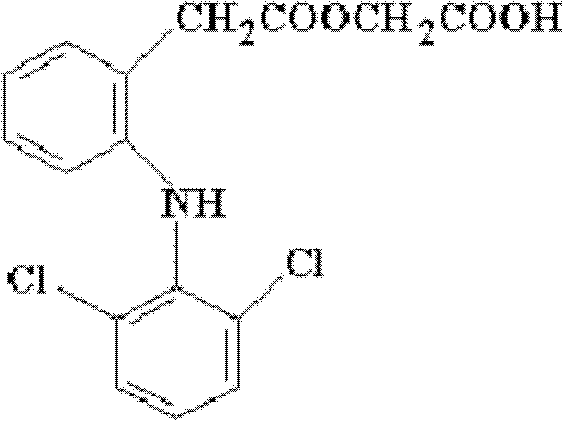
Aceclofenac-containing controlled-release oral drug preparations and their manufacturing process
A technology for aceclofenac and a control agent, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, and medical preparations without active ingredients, etc., can solve the problems of undeveloped preparations, drug treatment guidance and inconvenient compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
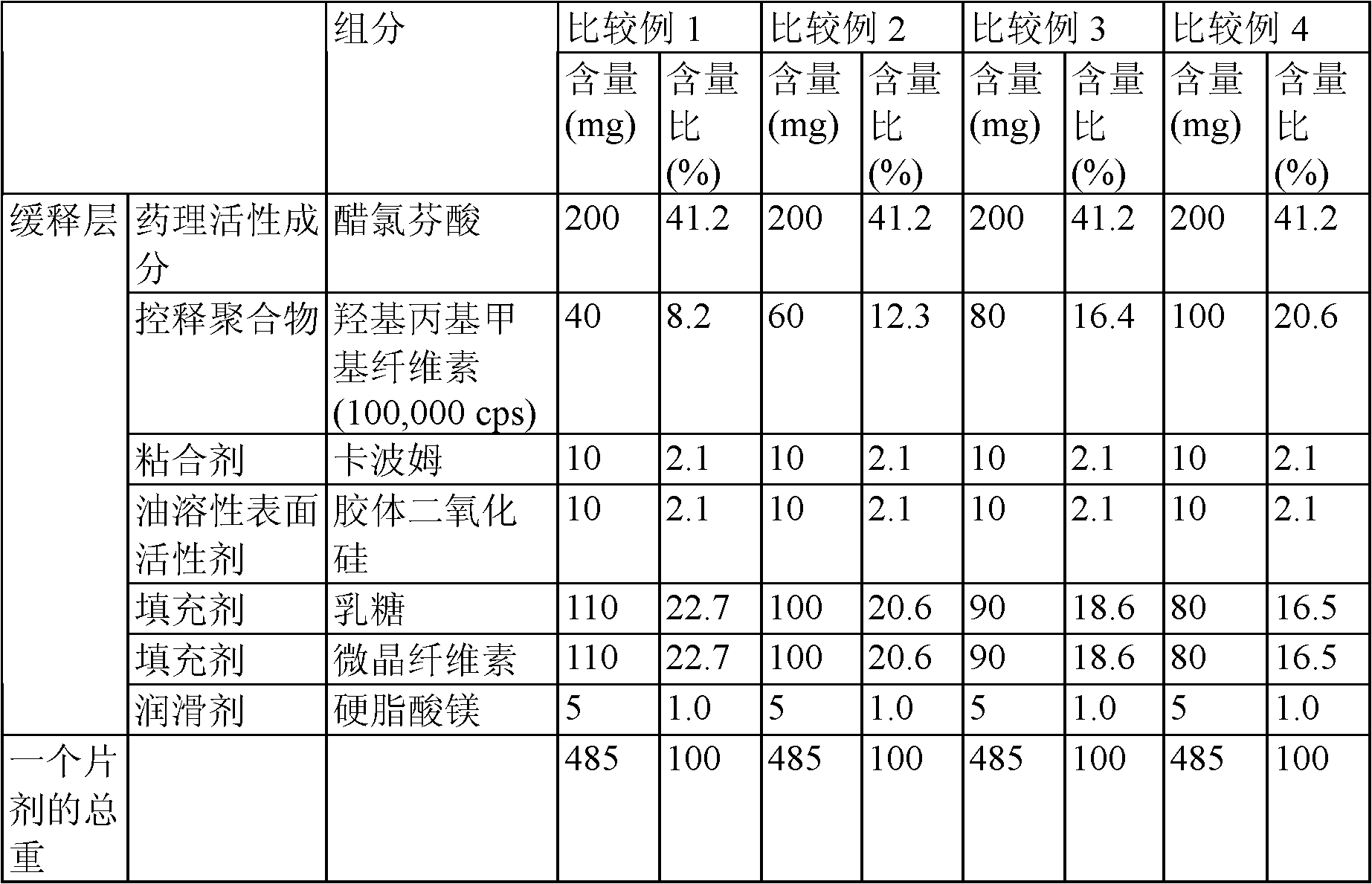
[0029] Water-soluble additives serve to additionally increase water absorption in the preparation of oral formulations to increase the initial release rate of the drug and enhance the absorption of the drug in the stomach.
[0030] Relative to the total weight of the aceclofenac sustained-release tablet, the weight ratio of the water-soluble additive is 0.75% to 3% by weight, preferably 1% to 2.5% by weight. If the weight ratio of the water-soluble additive is less than 0.75% by weight, the initial release rate of the drug will decrease; if it exceeds 3% by weight, the release rate of the drug will be excessively increased.
[0031]The disintegrant contained in the immediate-release layer of the aceclofenac sustained-release tablet serves to absorb water to facilitate the initial disintegration of aceclofenac and the dissolution of aceclofenac. Examples of disintegrants that may be used in the formulations of the invention include croscamellose sodium, sodium starch glycolate,...
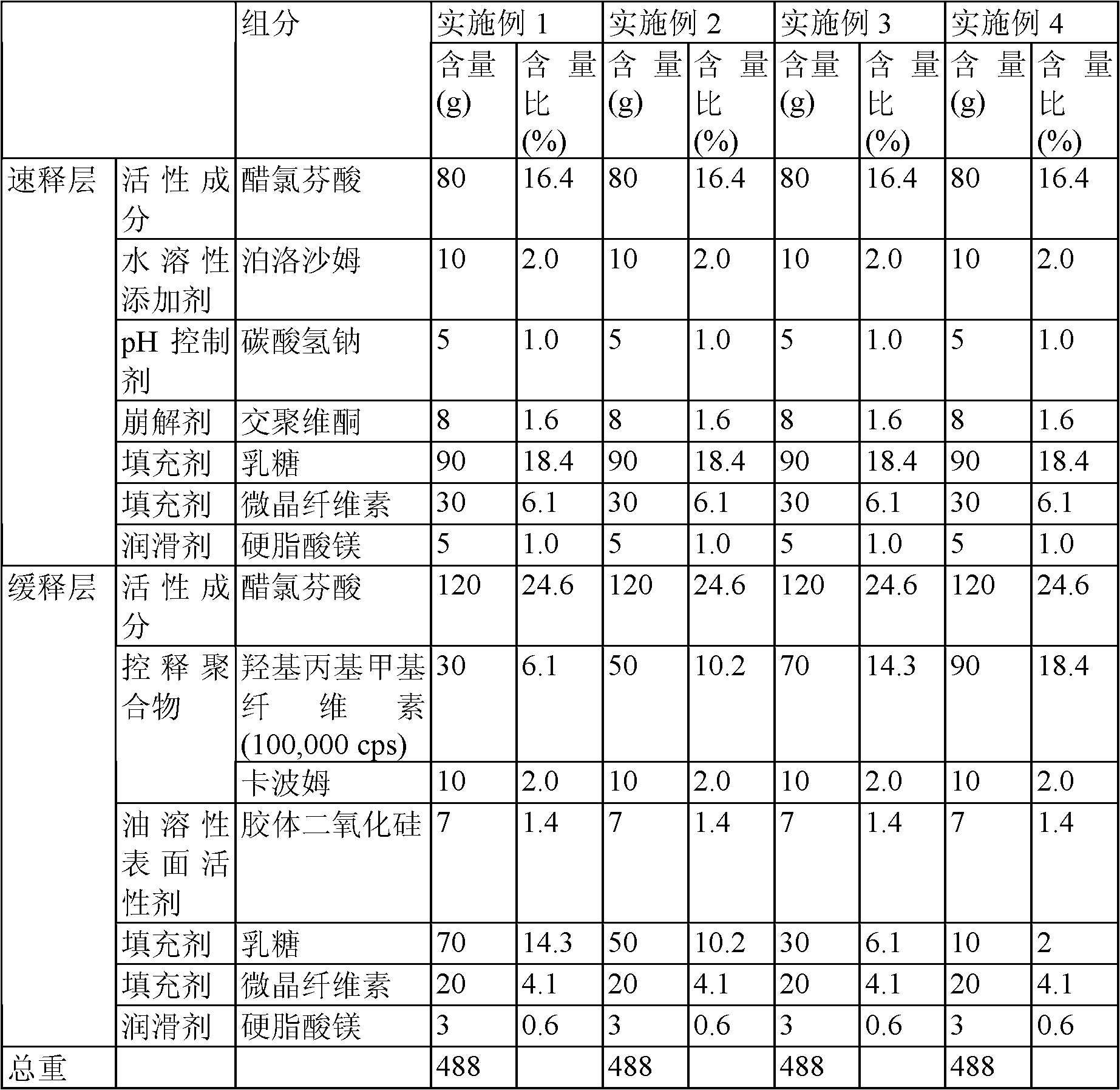
Embodiment 1~4
[0055] In the immediate-release layer, aceclofenac, lactose, microcrystalline cellulose, sodium bicarbonate, poloxamer, crospovidone, and magnesium stearate were mixed according to the components and contents shown in Table 1 below mixed with each other, thereby preparing immediate-release granules.
[0056]In the sustained-release layer, aceclofenac, lactose and microcrystalline cellulose are evenly mixed with each other to improve the fluidity of the drug. The mixture was uniformly mixed with hydroxypropylmethylcellulose (HPMC, 100,000 cps) and carbomer as a polymer base in a powder mixer, and then sprayed with ethanol, thereby preparing wet granules. The amounts of each component are shown in Table 1 below. Typically, 10 ml of ethanol is used to prepare 100 tablets. If necessary, a small amount of the polymer base material may be dissolved in water or a mixed solvent of water and alcohol and used to granulate the powder.
[0057] The prepared particles were fully dried i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com