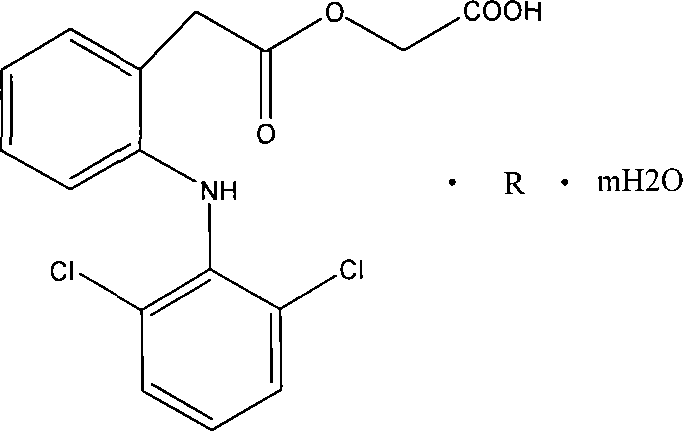
Salt compound formed by aceclofenac and organic base as well as composition and uses thereof
A salt compound, aceclofenac technology, applied in the direction of organic chemistry, drug combination, non-central analgesics, etc., can solve problems such as destruction, inability to take effect quickly, and pain relief for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
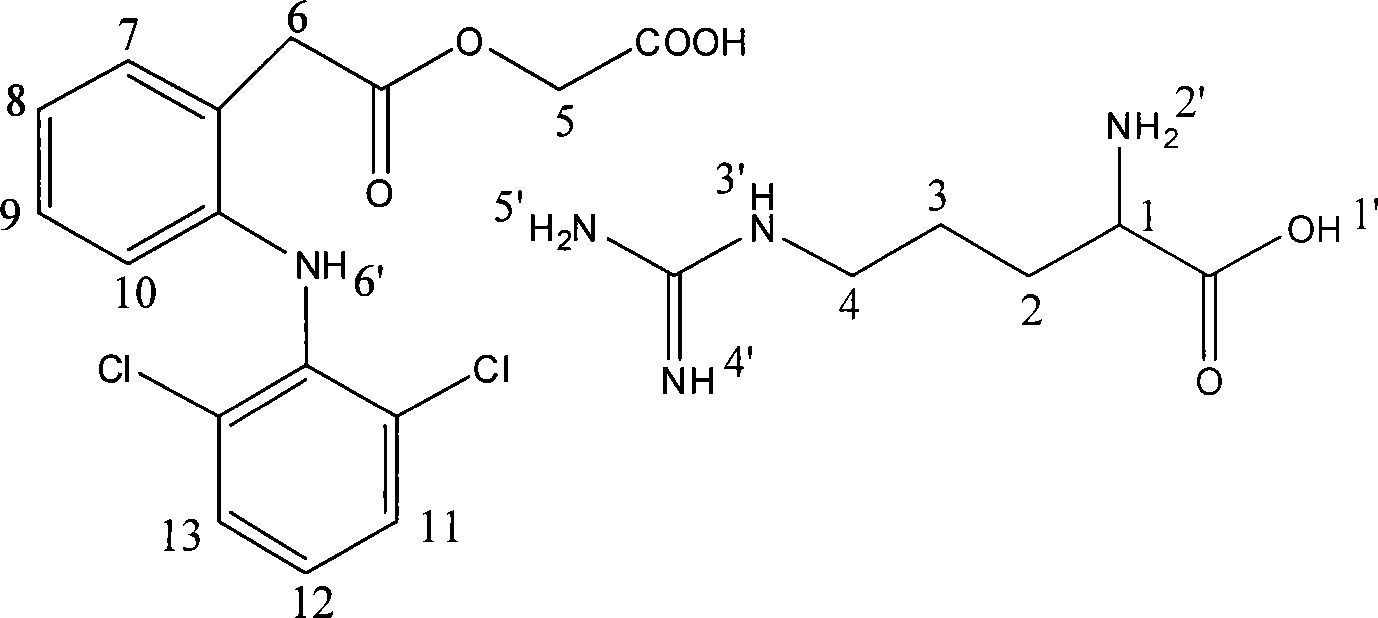
[0022] Example 1 Aceclofenac arginine (1:1, AR)
[0023] Dissolve 35.4 g (0.1 mol) of aceclofenac in 300 mL of ethanol (95%), heat and stir, slowly add 17.4 g (0.1 mol) of arginine in portions, heat to reflux for 30 min after the reaction solution is clear, cool and crystallize , precipitated solid, filtered, washed the filter cake with cold ethanol, and dried in an oven at 60°C to obtain 48.4g of the product.
[0024]
[0025] 1HNMR (DMSO 12 ): 1.56(2H, m, 3#), 1.68(2H, q, 2#), 3.03(2H, t, 4#), 3.29(1H, t, 1#), 3.82(2H, s, 6# ), 4.26(2H, s, 5#), 6.25(1H, d, 10#), 6.85(1H, t, 8#), 7.06(1H, d, 7#), 7.18(1H, t, 12# ), 7.22(1H, t, 9#), 7.29(1H, s, 6'), 7.51(2H, d, 11#, 13#), 8.0(5H, overlapping, 2'(2), 3', 4', 5' (1)).
example 2
[0026] Example 2 Aceclofenac arginine (2:1, A 2 R)
[0027] Dissolve 35.4g (0.1mol) of aceclofenac in 150mL of ethanol (95%), heat and stir, slowly add 8.7g (0.05mol) of arginine in portions, heat to reflux for 30min after the reaction solution is clear, add isopropyl 350ml of alcohol was cooled and crystallized, and a solid was precipitated, filtered, the filter cake was washed with cold ethanol, and dried in an oven at 60°C to obtain 39.3g of the product.
[0028]
[0029] 1HNMR (DMSO+D 2 O): 1.54(2H, m, 3#), 1.68(2H, q, 2#), 3.06(2H, t, 4#), 3.36(1H, t, 1#), 3.83(4H, s, 6 #), 4.43(4H, s, 5#), 6.23(2H, d, 10#), 6.86(2H, t, 8#), 7.06(2H, t, 7#), 7.17(2H, t, 12 #), 7.21 (2H, t, 9#), 7.48 (4H, d, 11#, 13#).
example 3
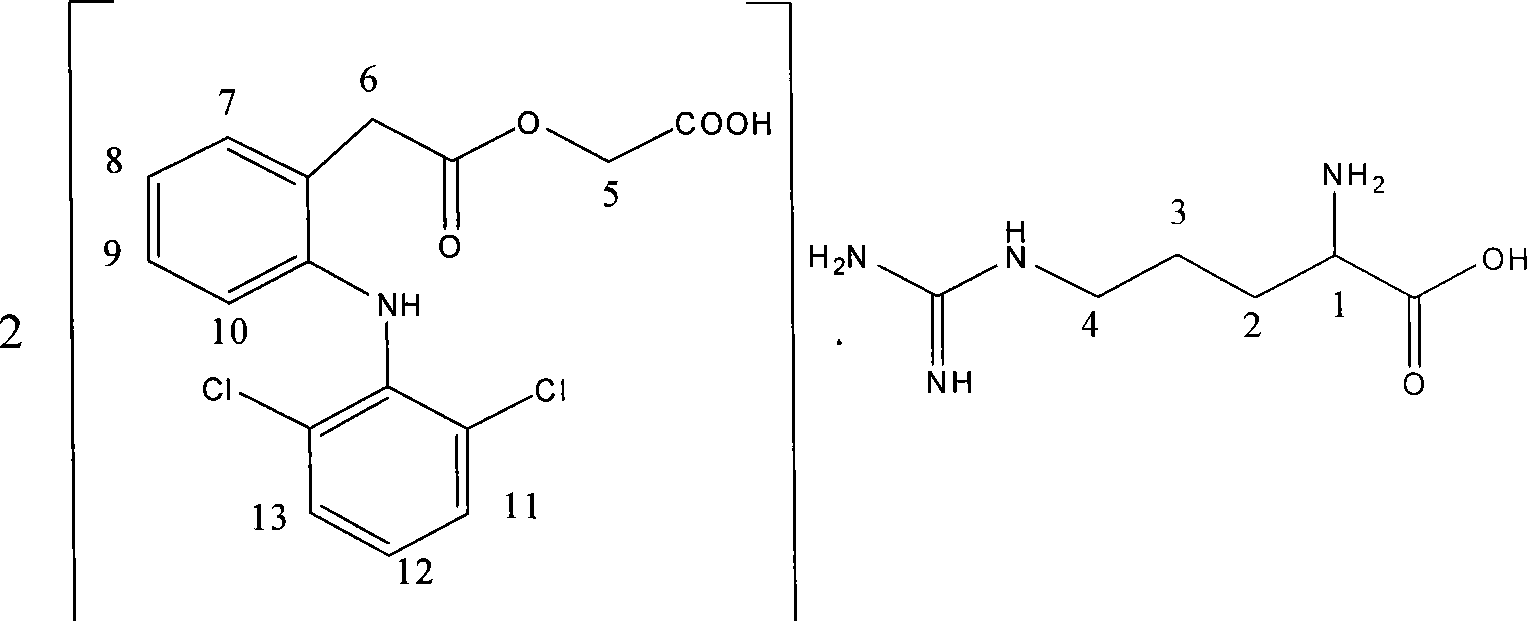
[0030] Example 3 Aceclofenac lysine
[0031] Dissolve 35.4g (0.1mol) of aceclofenac in a mixture of 200mL ethanol (95%) and 80ml water, heat and stir, slowly add 14.6g (0.1mol) of lysine in portions, and heat after the reaction solution becomes clear. Reflux for 30 minutes, cool and crystallize, precipitate solids, filter, wash the filter cake with cold ethanol, and dry in an oven at 60°C to obtain 46.3 g of the product.
[0032]
[0033] 1HNMR (D 2 O): 1.434(2H, m, 3#), 1.63(2H, m, 4#), 1.83(2H, q, 2#), 2.93(2H, t, 5#), 3.69(1H, t, 1 #), 3.79(2H, s, 7#), 4.38(2H, s, #6), 6.18(1H, d, 11#), 6.75(1H, t, 9#), 6.78(1H, d, 8 #), 6.82 (1H, t, 13#), 7.12 (1H, t, 10#), 7.10 (2H, d, 12#, 14#).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com