Aceclofenac pharmaceutical composition
A technology of aceclofenac and composition, applied in the field of aceclofenac pharmaceutical composition, can solve the problems of inconvenience in production and clinical use, hindering calcium and iron absorption, affecting calcium metabolism and hormone secretion, etc. The effect of financial burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
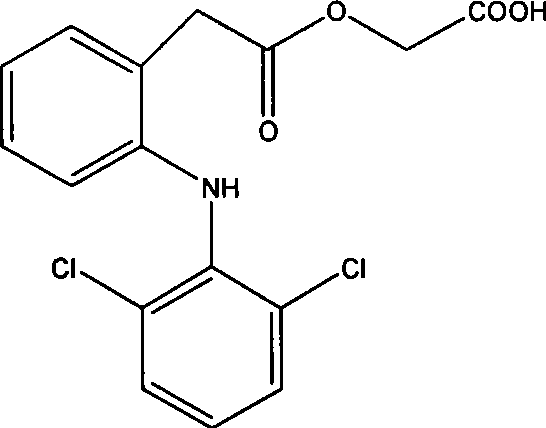
[0050] Example 1 Injection prescription 1
[0051]
[0052] Take by weighing aceclofenac, arginine of prescription quantity, stir and be dissolved in 2000ml of water for injection, pH is about 7.0, add needle and use active carbon 0.05% (W / V) to adsorb 30 minutes, carbon removal, sterilization filtration (0.22 μm) are subpackaged under sterile conditions, freeze-dried (moisture content ≤ 2%), sealed and capped.
[0053] When this product is used clinically, it can be directly dissolved in physiological saline and then injected without additional special solvent.
example 2
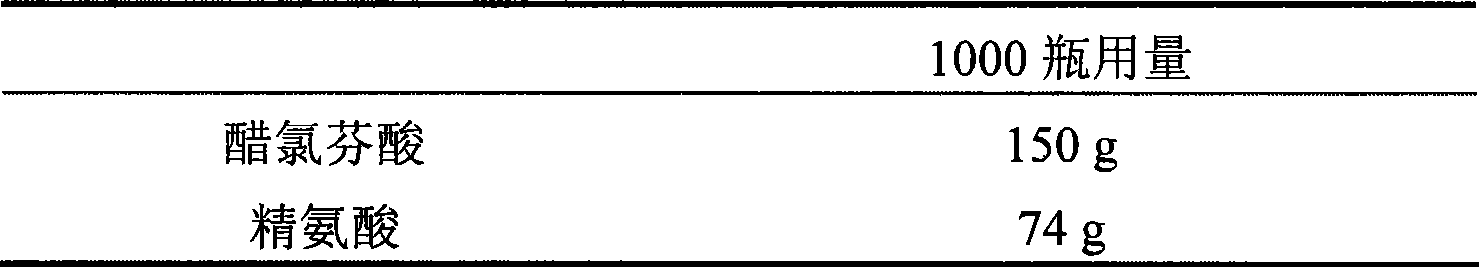
[0054] Example 2 injection prescription 2
[0055]
[0056]
[0057] Take by weighing aceclofenac, arginine, sodium chloride of prescription quantity, stir and be dissolved in 2000ml of water for injection, pH is about 7.0, add needle and use active carbon 0.05% (W / V) to adsorb for 30 minutes, remove charcoal, remove Bacterial filtration (0.22 μm) is subpackaged under sterile conditions, freeze-dried (moisture content ≤ 2%), sealed and capped.
[0058] When this product is used clinically, it can be directly dissolved in water for injection and then injected without additional special solvent.
example 3
[0059] Example 3 Injection prescription 3
[0060]
[0061] Aceclofenac and Potassium Citrate Monohydrate of prescription quantity are taken by weighing, mix in water for injection 3000ml, add 1M sodium hydroxide solution and adjust pH to be 6.9, add needle and use gac 0.05% (W / V) to adsorb 30 minutes, remove Charcoal, sterilizing filter (0.22μm), subpackage under aseptic conditions, freeze-dry (moisture content ≤ 3%), seal and crimp the cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com