Aceclofenac slow-release preparation providing an optimum pharmacological clinical effect when administered once a day
A technology for aceclofenac and controlled-release preparations, applied in the field of controlled-release preparations and controlled-release oral preparations, can solve problems such as difficulty in regulating aceclofenac, and achieve the effects of reducing administration frequency, rapid analgesic and anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
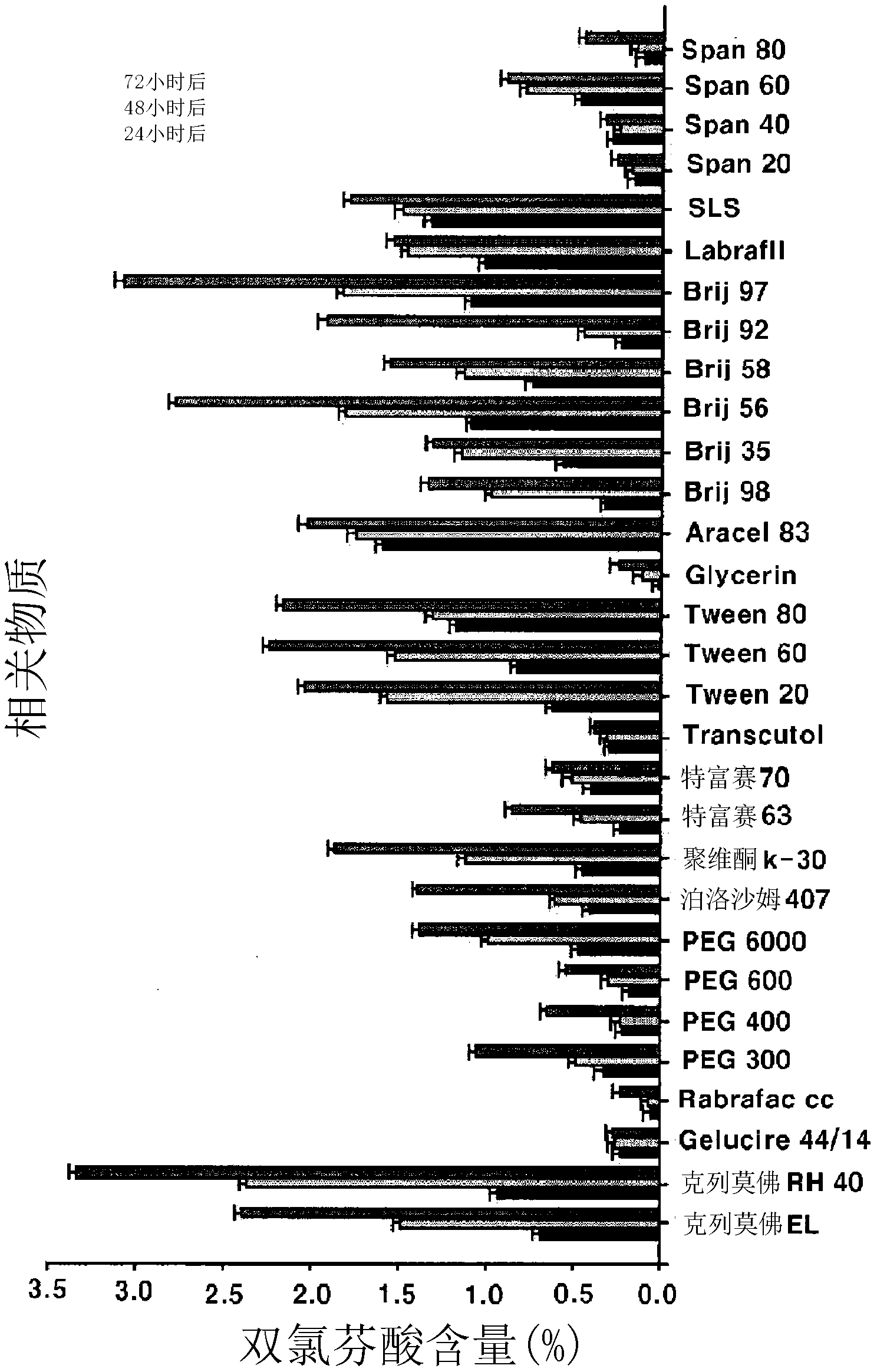
[0071] To confirm the interaction and compatibility between aceclofenac and excipients, the following tests were performed:
[0072] After manually mixing 200 mg of aceclofenac with each excipient, an accelerated test was performed at room temperature for 1 hour, and the mixture was allowed to stand for 1 month in an atmosphere with a humidity of 75% for content testing and softening substance testing. The test results are shown in Table 1.
[0073] [Table 1]
[0074]
experiment example 2
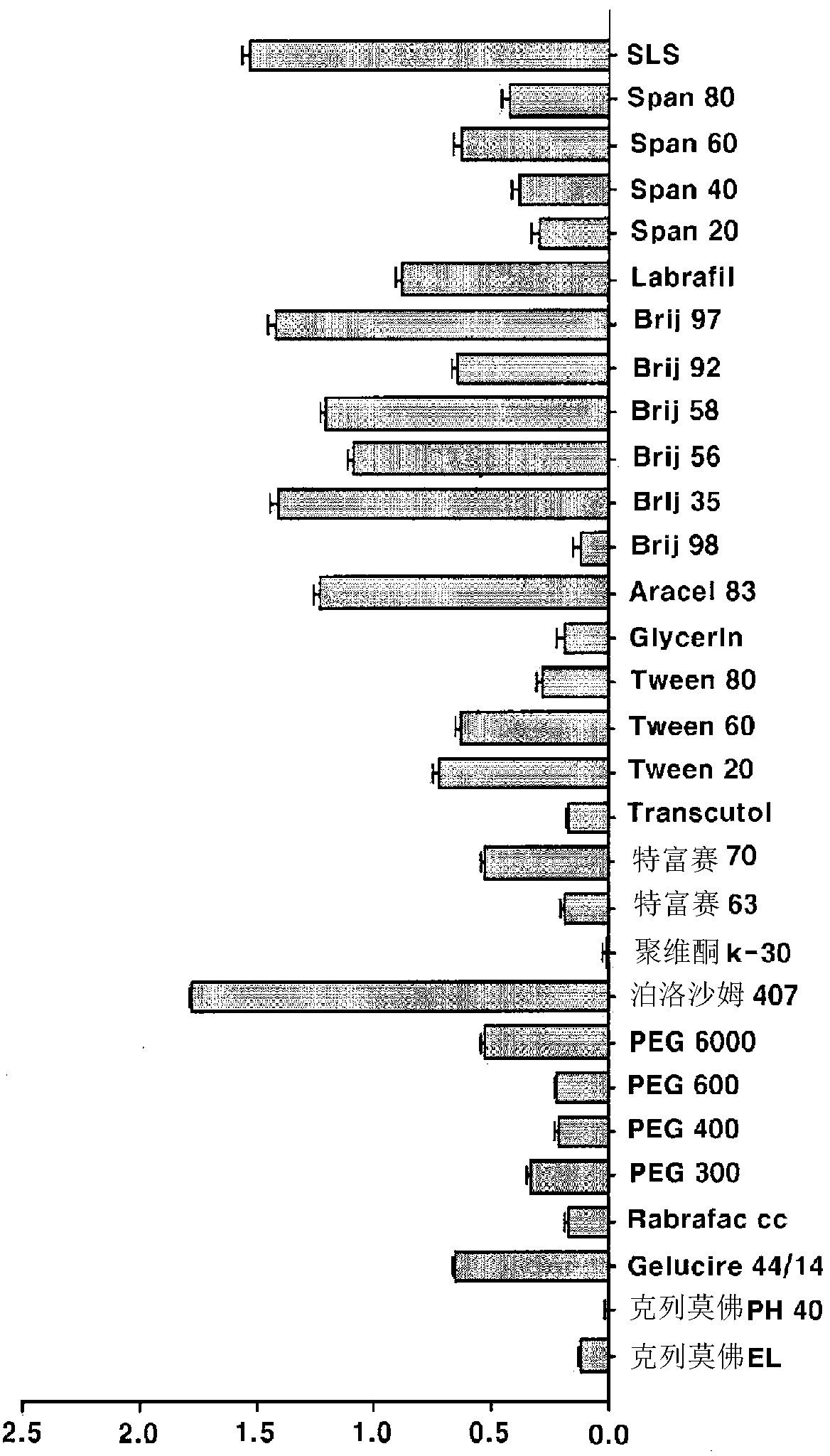
[0076] The degree of solubilization of aceclofenac was measured by the following test method.
[0077] 20 mg of aceclofenac was dispersed in 40 ml of water and then dissolved by adding 10 mg of a solubilizing agent. The aceclofenac suspension was allowed to stand at room temperature for 24 hours and then filtered with a 0.45 μm membrane filter. Then, the filtrate was passed through HPLC to confirm the solubility and softening of aceclofenac. The results are shown in Table 2.
[0078] UV detector: Jasco UV-975
[0079] Wavelength: 282nm
[0080] Column: Haisil ODS 15cm*4.6mm
[0081] Mobile phase: -65%MeOH:35%0.02M Potassium dihydrogen phosphate
[0082] Flow rate: 1.0ml / min
[0083] Injection volume: 20μl
[0084] [Table 2]
[0085]
[0086] The results of adding aceclofenac to each excipient solution (mg / ml) (n=3, mean ± S.D.) for confirming the softening substance of aceclofenac are shown in Table 3.
[0087] [table 3]
[0088]
[0089] The % of related subst...
experiment example 3
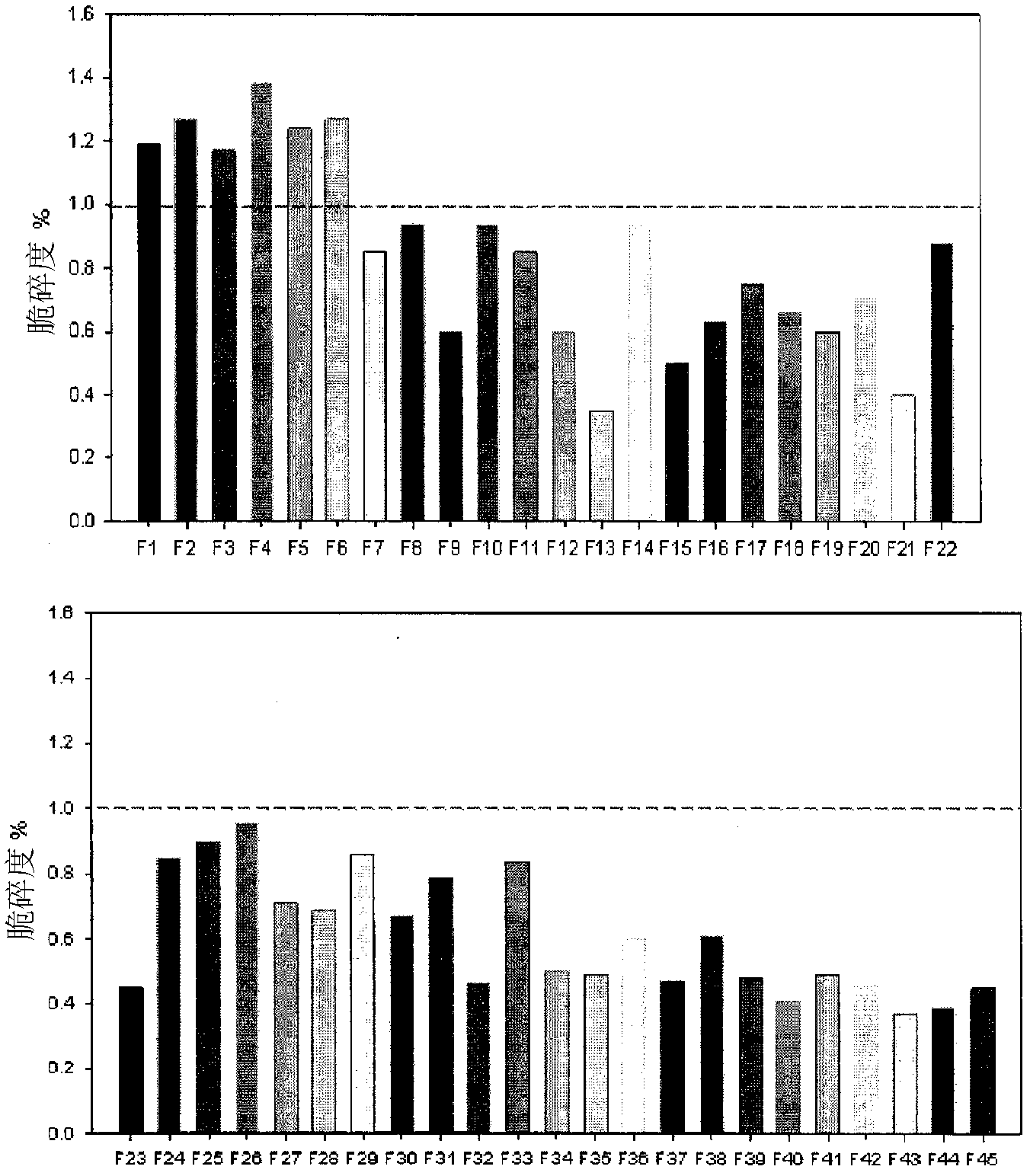
[0091] Formation of the immediate-release layer portion was determined by measuring physical properties between aceclofenac and each excipient.
[0092] -testing method
[0093] A fluidity test, a hardness test, and a friability test were performed on a mixture prepared by mixing aceclofenac with each of the excipients and then mixed with a lubricant to select suitable excipients. The test results are shown in Table 4.
[0094] [Table 4]
[0095]
[0096]
[0097]
[0098]
[0099]
[0100]
[0101] According to the test results, in terms of physical properties, crospovidone and poloxamer are the most suitable disintegrants and solubilizers, respectively, and the ratio of these two components is most preferably 1.2:1 to 1.7:1. It can be noted that when these two ingredients are included beyond the above mentioned ratios, the formulation is not suitable in terms of dissolution rate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com