Compound enteric-coated preparation and preparation method thereof
An enteric-coated preparation and compound technology, which is applied in the field of aceclofenac acetaminophen enteric-coated compound oral solid preparation and preparation, to achieve the effects of reducing gastric irritation, accurate drug content in the intestine, and the best curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
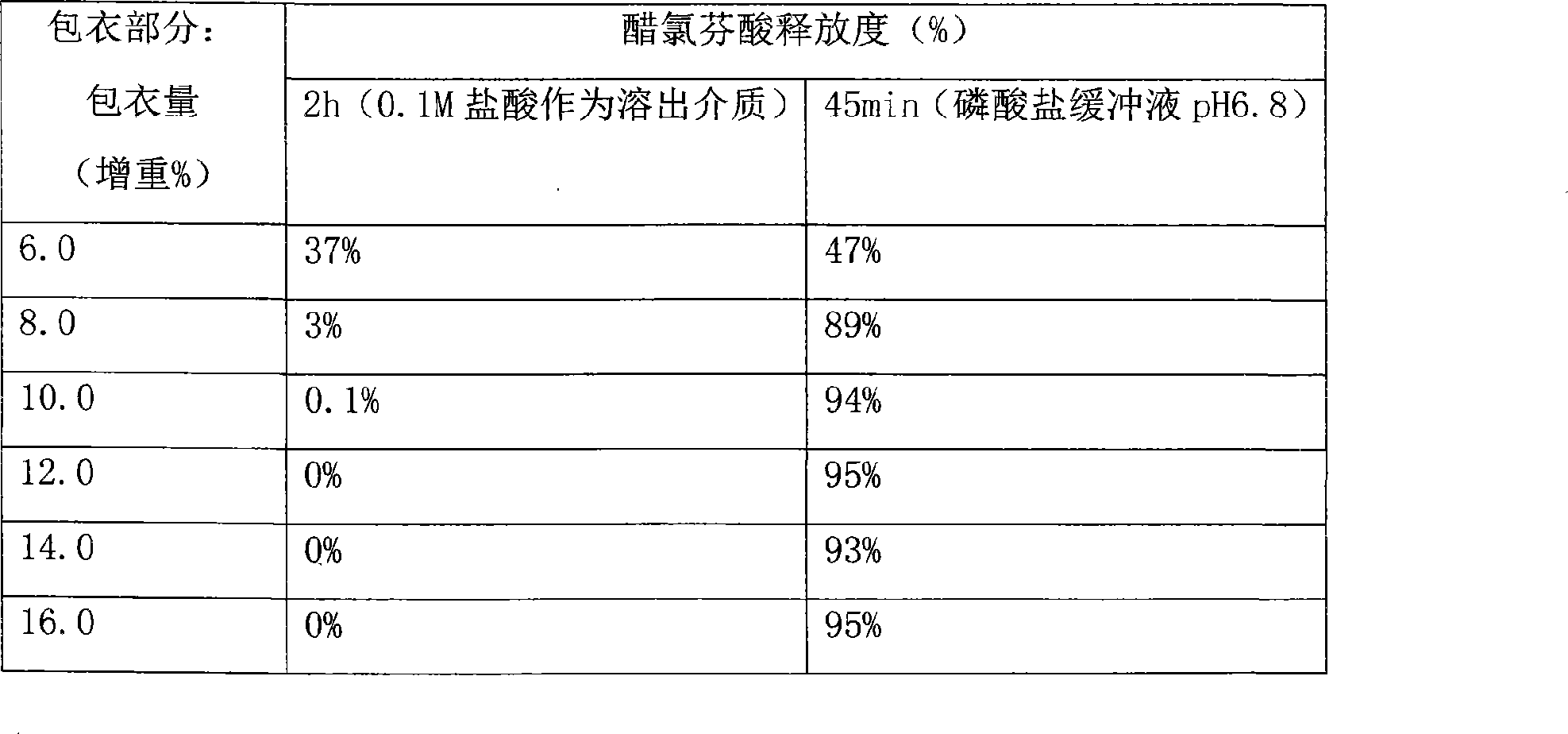
[0041] A enteric-coated part:
[0042] Aceclofenac Sodium 100g
[0043] Microcrystalline Cellulose 100g
[0044] Croscarmellose Sodium 8g
[0045] 5% PVP K30 60% ethanol solution 80ml
[0047] Appropriate amount of enteric coating solution
[0048] Preparation
[0049] 1. Take the prescribed amount of aceclofenac sodium, microcrystalline cellulose, and croscarmellose sodium and mix evenly, 5% PVP K30 60% ethanol solution to make pellets.
[0050] 2. Coating until the weight gain is about 13%.
[0051] B quick release part
[0052] Acetaminophen 500g
[0054] Preparation
[0055] The acetaminophen and magnesium stearate are mixed well.
[0056] C Finished product preparation
[0057] Preparation
[0058] Mix the enteric-coated part and the immediate-release part evenly, and pack into capsules.
Embodiment 2
[0060] A enteric-coated part:
[0061] Aceclofenac Sodium 100g
[0062] Microcrystalline Cellulose 100g
[0063] Croscarmellose Sodium 8g
[0064] 5% PVP K3060% ethanol solution 80ml
[0066] Appropriate amount of enteric coating solution
[0067] Preparation
[0068] 1. Take the prescribed amount of aceclofenac sodium, microcrystalline cellulose, and croscarmellose sodium and mix evenly, 5% PVP K30 60% ethanol solution to make pellets.
[0069] 2. Coating until the weight gain is about 13%.
[0070] B quick release part
[0071] Acetaminophen 500g
[0072] Hydroxypropyl Cellulose 80
[0073] 5% PVP K30 60% ethanol solution 150ml
[0074] Preparation
[0075] Acetaminophen and hydroxypropyl cellulose mixed well, 5% PVP K30 60% ethanol solution to make granules.
[0076] C Finished product preparation
[0077] Preparation
[0078] Mix the enteric-coated part and the immediate-release part evenly, add a small amount of lubricant, and pac...
Embodiment 3
[0080] A enteric-coated part:
[0081] Aceclofenac Sodium 100g
[0082] Microcrystalline Cellulose 100g
[0083] Croscarmellose Sodium 8g
[0084] 5% PVP K30 60% ethanol solution 80ml
[0086] Appropriate amount of enteric coating solution
[0087] Preparation
[0088] 1. Take the prescribed amount of aceclofenac sodium, microcrystalline cellulose, and croscarmellose sodium, mix them evenly, and make pellets with 5% PVPK30 and 60% ethanol solution.
[0089] 2. Coating until the weight gain is about 13%.
[0090] B quick release part
[0091] Acetaminophen 500g
[0092] Microcrystalline Cellulose 200g
[0093] 5% PVP K30 60% alcohol solution 300ml
[0094] Preparation
[0095] Acetaminophen and microcrystalline cellulose mixed well, 5% PVP K30 60% ethanol solution to make pellets.
[0096] C Finished product preparation
[0097] Preparation
[0098] Mix the enteric-coated part and the immediate-release part evenly, add a small amount of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com