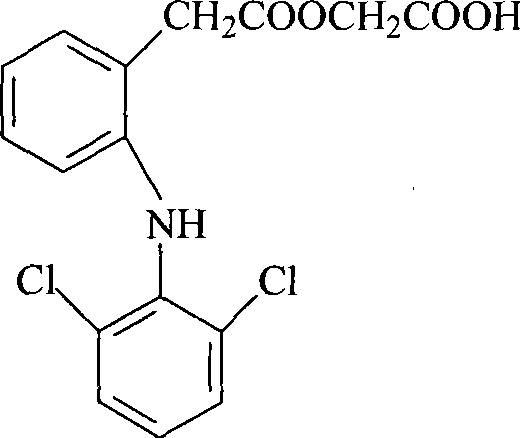
Improved method for preparing aceclofenac
A technology of aceclofenac and diclofenac sodium, which is applied to the preparation of cyanide reaction, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of easy falling off of protective groups, acidolysis selectivity, low yield, etc., to avoid Effects of side reactions, improvement of product yield and purity, and improvement of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
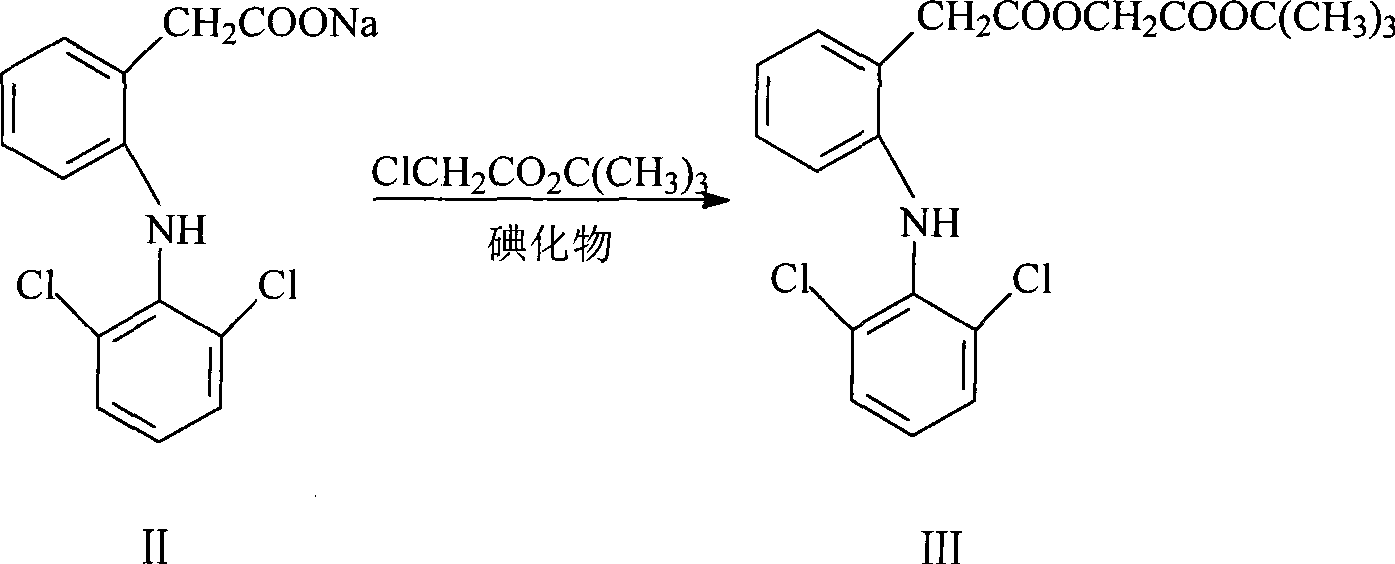
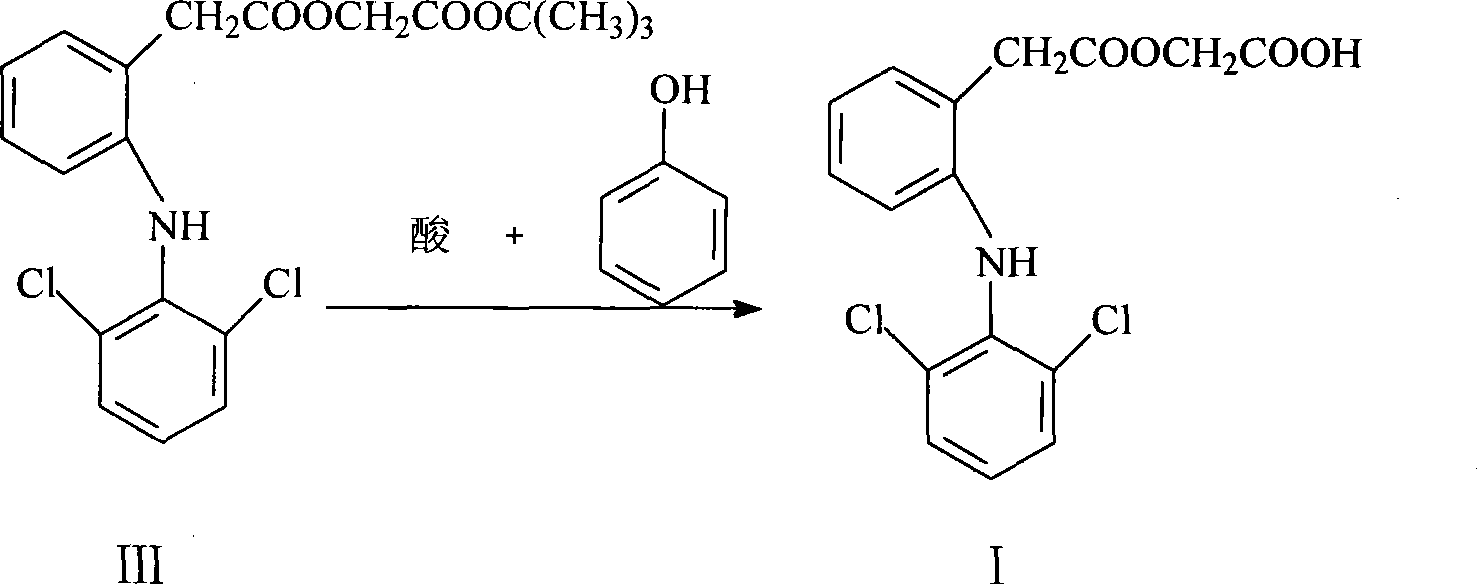
[0035] Example 1 Preparation of tert-butyl 2-(2,6-dichloroanilino)phenylacetoxyacetate (compound III)
[0036] Put 20 g of diclofenac sodium, 0.5 g of sodium iodide, 10.4 g of tert-butyl chloroacetate, and 100 ml of acetone into the reaction flask, heat to about 60° C. for reflux reaction for 2 hours, and spot the plate with TLC (developing agent: n-hexane: ethyl acetate = 6:1 (volume ratio)) After the reaction, filter out sodium chloride, recover acetone at 60-65°C under normal pressure, then evaporate the acetone to dryness under reduced pressure, add methanol to reflux to refine the intermediate, cool and crystallize, filter, and vacuum dry Oven drying (temperature 45°C-55°C, vacuum degree ≥ 0.09 MPa) yielded 25.0 g of white crystals (yield: 97.0%, mp: 87.8-88.2°C).
Embodiment 2
[0037] Example 2 Preparation of tert-butyl 2-(2,6-dichloroanilino)phenylacetoxyacetate (compound III)
[0038]Put 20 g of diclofenac sodium, 0.6 g of potassium iodide, 11.3 g of tert-butyl chloroacetate, and 200 ml of acetone into the reaction bottle, heat it to about 60° C. for reflux reaction for 2.5 hours, and spot the plate with TLC (developing agent: n-hexane: ethyl acetate=6: 1 (volume ratio)) After the reaction, remove sodium chloride by filtration, recover acetone under normal pressure at 60-65°C, then evaporate acetone to dryness under reduced pressure, add methanol to reflux to refine the intermediate, cool and crystallize, filter, and dry in a vacuum oven (45°C-55°C, vacuum degree ≥0.09 MPa), 24.3 g of white crystals were obtained (yield: 94.2%, mp: 87.6-88.2°C).
Embodiment 3
[0039] Example 3 Preparation of tert-butyl 2-(2,6-dichloroanilino)phenylacetoxyacetate (compound III)
[0040] Put 20 g of diclofenac sodium, 0.5 g of sodium iodide, 10.4 g of tert-butyl chloroacetate, and 80 ml of DMF into the reaction bottle, heat it to about 80 ° C for 1.5 hours, and spot the plate with TLC (developing agent: n-hexane: ethyl acetate = 6: 1 (volume ratio)) After the reaction, filter out sodium chloride, evaporate DMF under reduced pressure, add methanol to reflux to refine the intermediate, cool and crystallize, filter, and dry in a vacuum oven (45 ° C ~ 55 ° C, vacuum degree ≥ 0.09MPa), to obtain 24.8g of white crystals (yield: 96.2%, mp: 87.3-87.7°C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com