Aceclofenac enteric-coated pellet capsule and preparation method thereof
A technology of enteric-coated pellets and aceclofenac, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, can solve problems such as indigestion, gastrointestinal irritation, and abdominal discomfort, and achieve guaranteed The effects of roundness, stability and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 Preparation of aceclofenac enteric-coated pellet capsules of the present invention
[0071] The prescription is composed of the following components by weight (unit: mg):
[0072] a. Blank ball core
[0073] Sucrose 35
[0074] starch 20
[0075] Microcrystalline Cellulose 15
[0076] b. The main drug layer
[0077]
[0078] c. Isolation layer
[0079] Micronized silica gel 18
[0080] Hypromellose E5 2
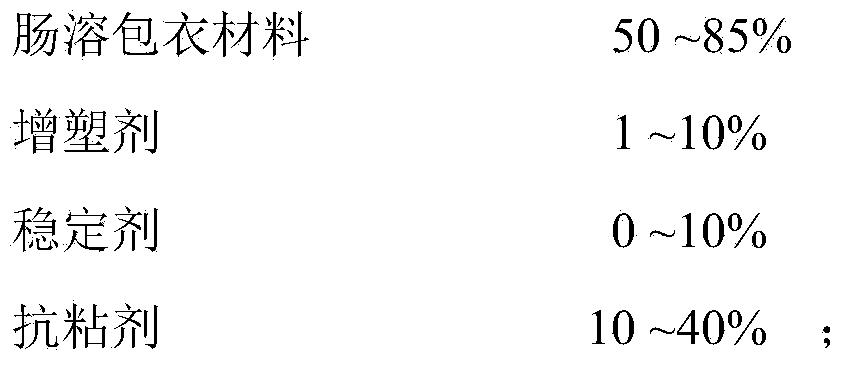
[0081] d. Enteric coating layer
[0082]
[0083] Preparation:
[0084] (1) Preparation of blank ball cores: prepare 50% sucrose aqueous solution as a binder, and prepare blank ball cores by weighing the prescribed amount of starch and / or microcrystalline cellulose, and set aside;
[0085] (2) Wrapping the main drug layer: take the prescribed amount of emulsifier, and prepare it into a 5% emulsifier aqueous solution. At a temperature of 20°C and a stirring speed of 400 to 1200 rad / min, add the prescribed amount of disintegrant, Pre-emulsify...
Embodiment 2
[0089] Embodiment 2 Preparation of aceclofenac enteric-coated pellet capsules according to the present invention
[0090] The prescription is composed of the following components by weight (unit: mg):
[0091] a. Blank ball core
[0092] Sucrose 35
[0093] Microcrystalline Cellulose 35
[0094] b. The main drug layer
[0095]
[0096] c. Isolation layer
[0097] Micronized silica gel 12
[0098] Hypromellose E5 3
[0099] d. Enteric coating layer
[0100]
[0101] Preparation:
[0102] (1) Preparation of blank ball cores: prepare 50% sucrose aqueous solution as a binder, and prepare blank ball cores by weighing the prescribed amount of starch and / or microcrystalline cellulose, and set aside;
[0103] (2) Wrapping the main drug layer: take the prescribed amount of emulsifier, and prepare it into a 5% emulsifier aqueous solution. At a temperature of 20°C and a stirring speed of 400 to 1200 rad / min, add the prescribed amount of disintegrant, Pre-emulsify for 0.5h ...
Embodiment 3
[0107] Embodiment 3 Preparation of aceclofenac enteric-coated pellet capsules according to the present invention
[0108] The prescription is composed of the following components by weight (unit: mg):
[0109] a. Blank ball core
[0110] Sucrose 30
[0111] Starch 50
[0112] b. The main drug layer
[0113]
[0114] Monoglyceride diacetyl tartrate 10
[0115] c. Isolation layer
[0116] Micronized silica gel 20
[0117] Hypromellose E5 4
[0118] d. Enteric coating layer
[0119]
[0120] Preparation:
[0121] (1) Preparation of blank ball cores: prepare 50% sucrose aqueous solution as a binder, and prepare blank ball cores by weighing the prescribed amount of starch and / or microcrystalline cellulose, and set aside;
[0122] (2) Wrapping the main drug layer: take the prescribed amount of emulsifier, and prepare it into a 5% emulsifier aqueous solution. At a temperature of 20°C and a stirring speed of 400 to 1200 rad / min, add the prescribed amount of disintegrant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com