Method for performing headspace gas chromatographic detection on formic acid in aceclofenac bulk pharmaceutical chemicals
A headspace gas chromatography and aceclofenac technology, applied in the field of formic acid headspace gas chromatography detection, can solve problems such as human body and environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
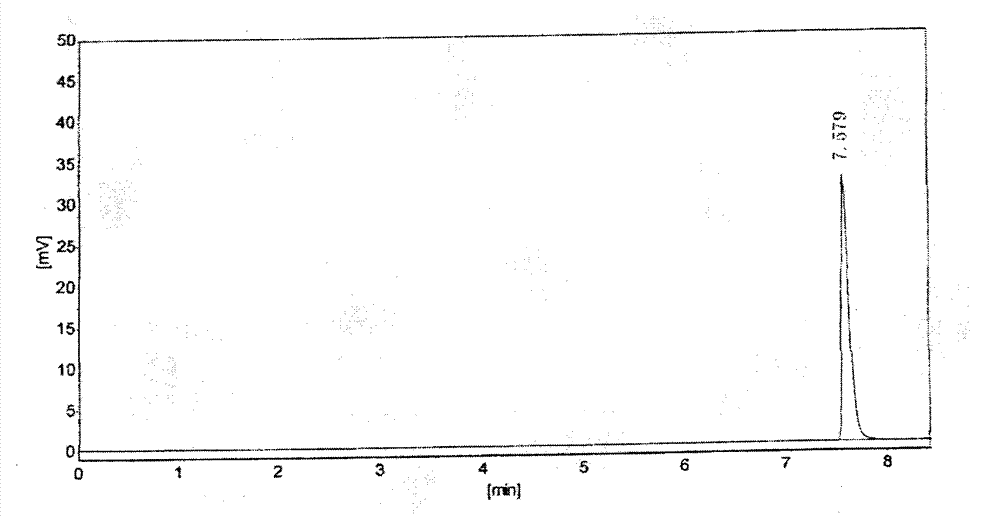
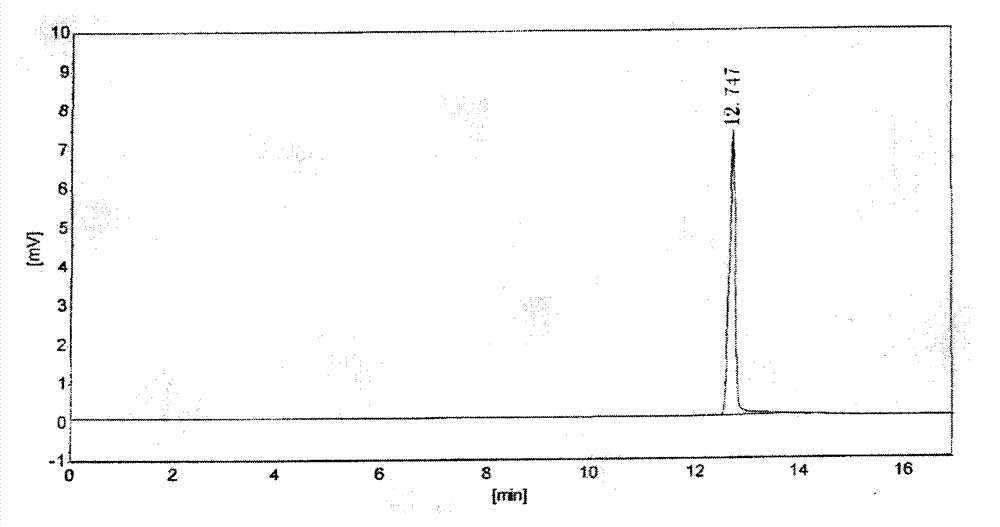
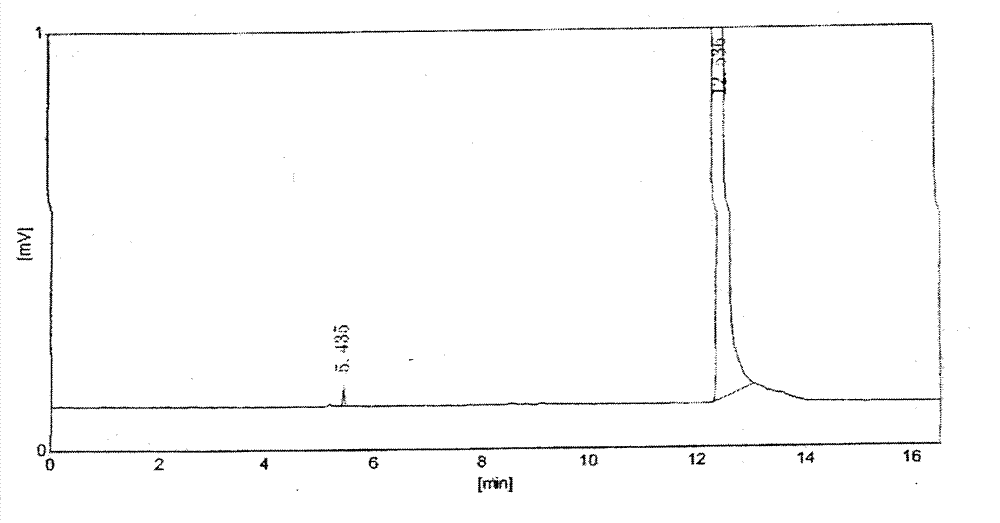
[0022] Get about 0.15g of aceclofenac crude drug, accurately weighed, add 10% (v / v) sulfuric acid-methanol solution 1ml, put into 40 ℃ water bath, after reacting for 20min, it will be used as the test solution; About 0.1mg, accurately weighed, add 1ml of 10% (v / v) sulfuric acid-methanol solution, put it in a water bath at 40°C, and react for 20min, then use it as the reference solution. The test solution and the reference solution were analyzed by headspace injection respectively, the chromatograms were recorded, and the content of formic acid in the test was calculated by the external standard method.
Embodiment 2
[0024] Take about 0.15g of aceclofenac raw material, weigh it accurately, add 0.5ml of anhydrous methanol to disperse and dissolve, then add 0.5ml of 20% (v / v) sulfuric acid-methanol solution, put it in a water bath at 40°C, and react for 20min , that is, as the test solution; take about 0.1mg of formic acid, accurately weighed, add 0.5ml of anhydrous methanol to disperse and dissolve, then add 0.5ml of 20% (v / v) sulfuric acid-methanol solution, and put it in a 40°C water bath , After 20min of reaction, it was used as the reference solution. The test solution and the reference solution were analyzed by headspace injection respectively, the chromatograms were recorded, and the content of formic acid in the test was calculated by the external standard method.
[0025] The chromatographic conditions of gas chromatography are:
[0026] Chromatographic column: HP-FFAP capillary column (50m×0.32mm×0.5μm); column temperature: 40°C; carrier gas: nitrogen; flow rate: 3ml / min; inlet te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com