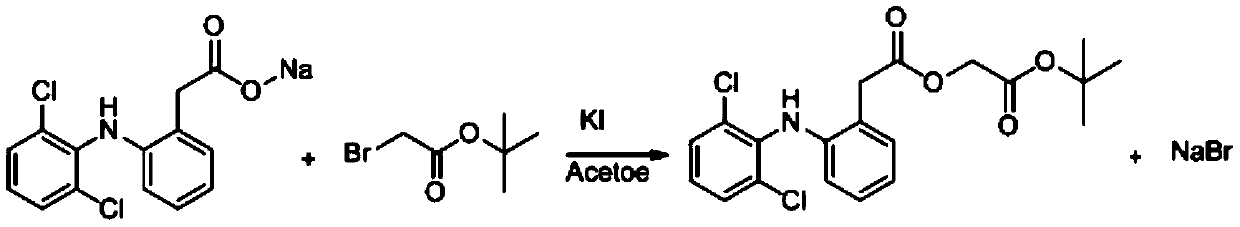
Method for producing aceclofenac tert-butyl ester
A technology of aceclofenac and tert-butyl bromoacetate, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc. It can solve the problems of difficult product separation, large discharge of organic waste solvents, and low process safety factor and other issues, to achieve the effect of good application prospect, sufficient response and safe process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 100g water in the there-necked flask of 250ml, start stirring, add 20g (80%, 50.31mmol) diclofenac sodium crude product (DOS5), add 16.7g (85.64mmol, 1.7eq) tert-butyl bromoacetate, 0.6g (3.614mmol , 0.07eq) potassium iodide, 1.6g (4.713mmol, 0.09eq) tetrabutylammonium bisulfate, turn on the heating, raise the temperature to 60°C, start to keep warm, control the temperature at 60-65°C, keep warm for 2 hours, slowly cool down to the temperature Keep warm at 5-15°C for half an hour, vacuum filter to obtain a brownish-yellow solid, wash the solid with ethanol 3 times the mass of DOS5 until off-white, and dry at 60°C for 30 hours to obtain 17.3 g of tert-butyl aceclofenac. The yield is 94.5%, and the liquid phase purity is 99.62%.
Embodiment 2
[0020] Add 500g water in the there-necked flask of 1000ml, start to stir, add 100g (80%, 251.6mmol) diclofenac sodium crude product (DOS5), add 73.6g (377.4mmol, 1.5eq) tert-butyl bromoacetate, 2.9g (17.61mmol , 0.07eq) potassium iodide, 7.7g (22.64mmol, 0.09eq) tetrabutylammonium bisulfate, turn on the heating, raise the temperature to 60°C, start to keep warm, control the temperature at 60-65°C, keep warm for 2.5 hours, slowly cool down to the temperature Keep warm at 5-15°C for half an hour, vacuum filter to obtain a brownish-yellow solid, wash the solid with ethanol 3 times the mass of DOS5 until off-white, and dry at 60°C for 30 hours to obtain 88.7g tert-butyl aceclofenac. The yield is 94.16%, and the liquid phase purity is 99.43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com