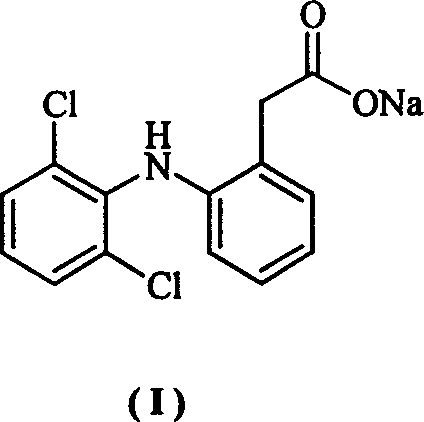
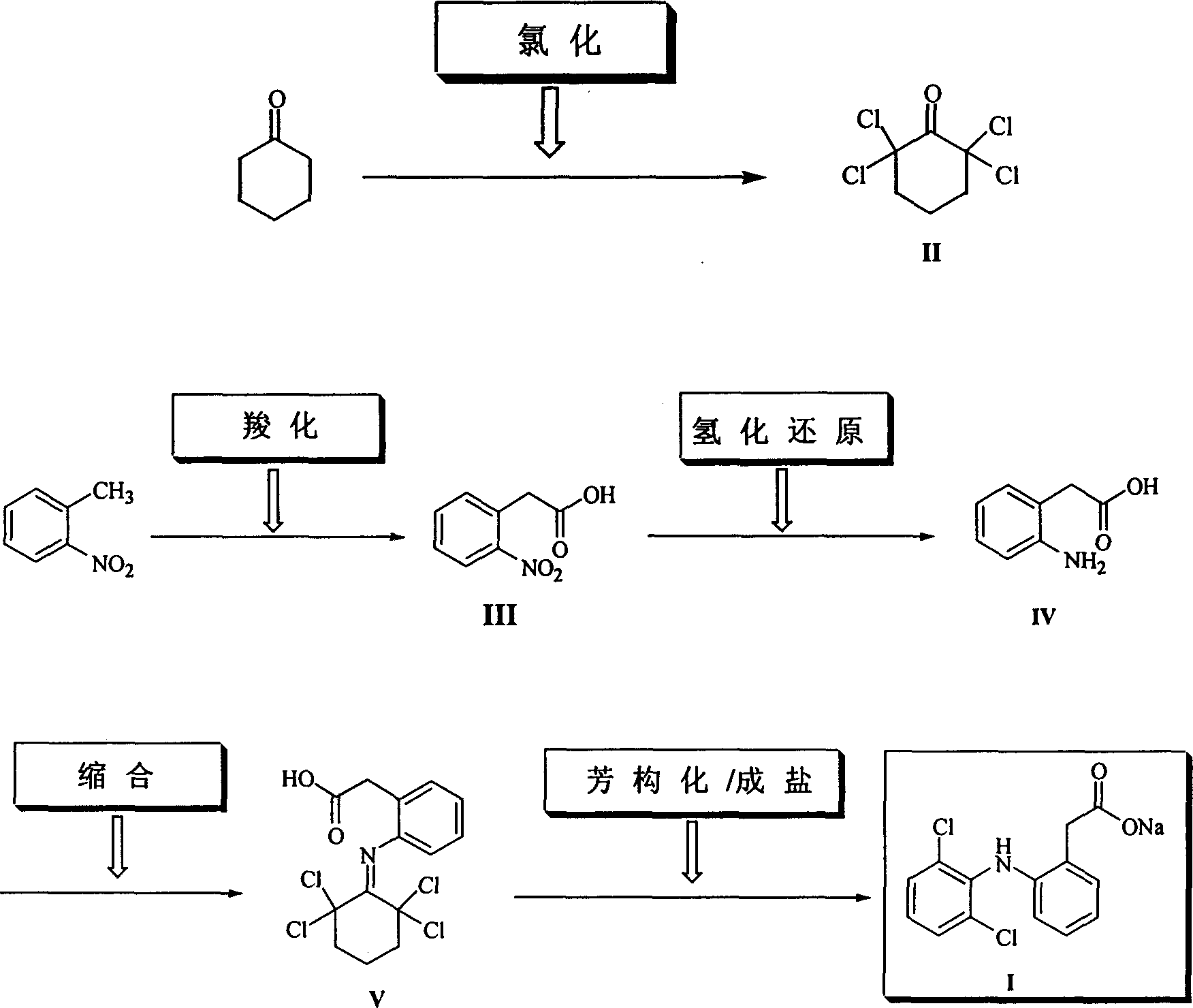
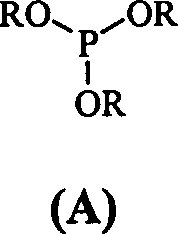Method for synthesizing dichlofenac sodium
A technology of diclofenac sodium and a synthesis method, applied in the field of medicinal chemistry, can solve the problems of serious three-waste pollution and high labor protection requirements, and achieve the effects of environmental friendliness, good industrialization prospects and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1 Cyclohexanone (50g, 0.51mol), cyclohexane (200mL) and triethyl phosphite (12g, 0.072mol) were placed in a dry reaction flask, and after heating and stirring to reflux, chlorine gas was slowly introduced until GLC showed a ring The hexanone starting material peaks all disappeared. When the content of II reaches 98%, stop flowing chlorine (consume about 185g of chlorine gas), and continue to stir and reflux for 40min. After the reaction was completed, it was cooled to 10°C, and a solid precipitated out, which was filtered. Wash with a small amount of cyclohexane and dry at 50°C to obtain white crystalline powder II (118g, 98%), mp 81-82°C.
example 2
[0024] Example 2 Cyclohexanone (50g, 0.51mol), petroleum ether (350mL) and triphenyl phosphite (1.58g, 0.0051mol) were placed in a dry reaction flask, and after heating and stirring to reflux, chlorine gas was slowly introduced until GLC showed a ring All peaks of the hexanone raw material disappeared, and when the content of compound (II) reached 98.5%, the introduction of chlorine gas was stopped, and stirring and reflux were continued for 30 min. After the reaction is complete, cool to 5-10°C, solid precipitates, and filter. Wash with a small amount of petroleum ether and dry at 50°C to obtain white crystalline powder II (111.9g, 94%), mp 79-81°C.
example 3
[0025] Example 3 Cyclohexanone (50g, 0.51mol), petroleum ether (350mL) and trimethyl phosphite (1.9g, 0.015mol) were placed in a dry reaction flask, and after heating and stirring to reflux, chlorine gas was slowly introduced until GLC showed a ring The peaks of the hexanone raw material all disappeared, and the chlorine gas was stopped, and the stirring and reflux were continued for 30 min. After the reaction was completed, it was cooled to 5°C, and a solid precipitated out, which was filtered. Wash with a small amount of petroleum ether and dry at 50°C to obtain white crystalline powder II (110.7g, 92%), mp 80-82°C.
[0026] Two, the preparation of o-nitrophenylacetic acid (III)
[0027]Example 1 Put o-nitrotoluene (41.2g, 0.30mol), sodium hydroxide (30g, 0.75mol), polyethylene glycol-600 (0.5g) and toluene (200mL) in a dry reaction flask, heat and stir to reflux for 1h , and then introduce carbon dioxide gas until no carbon dioxide is absorbed (about 6h, about 23.15g cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com