Sodium dichlorophenolate sustained-release tablet and method for controlling sustained-release of sodium dichlorophenolate sustained-release tablet
A technology of diclofenac sodium and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as retention and no release of diclofenac sodium sustained-release preparations, Achieve the effect of reducing rework, reducing energy consumption, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
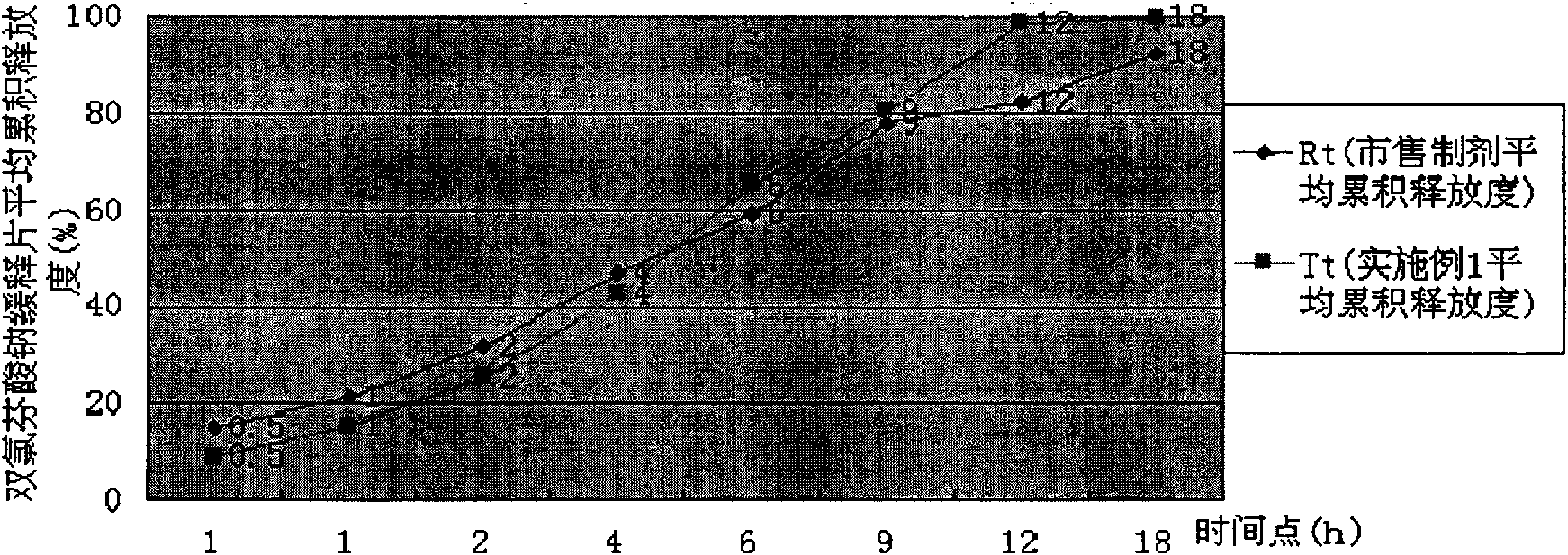
[0034] Take by weighing diclofenac sodium slow-release tablet powder 25g (wherein HPMC K4M CR accounts for 20% of powder gross weight), add 50g hot distilled water, glass rod stirs and makes it disperse, add distilled water 425g, high-shear mixing emulsifier fully stirs 40 minutes, Prepare an aqueous solution containing 1% HPMC, and use a Brookfield LVDV-C digital display viscometer to select the #2 rotor at a speed of 10 rpm to measure the viscosity to be 441 centipoise. The release rate of diclofenac sodium sustained-release tablets prepared at this time is shown in Table 1, and compared with the release rate of commercially available preparations, the dissolution curve similarity f2 factor method evaluation is shown in Table 1. figure 1 .
[0035] The release degree of table 1 embodiment 1
[0036]
[0037] Conclusion: Example 1 is evaluated according to the dissolution curve similarity f2 factor, f2=55>50, it is judged that its release is similar to the commerci...
Embodiment 2
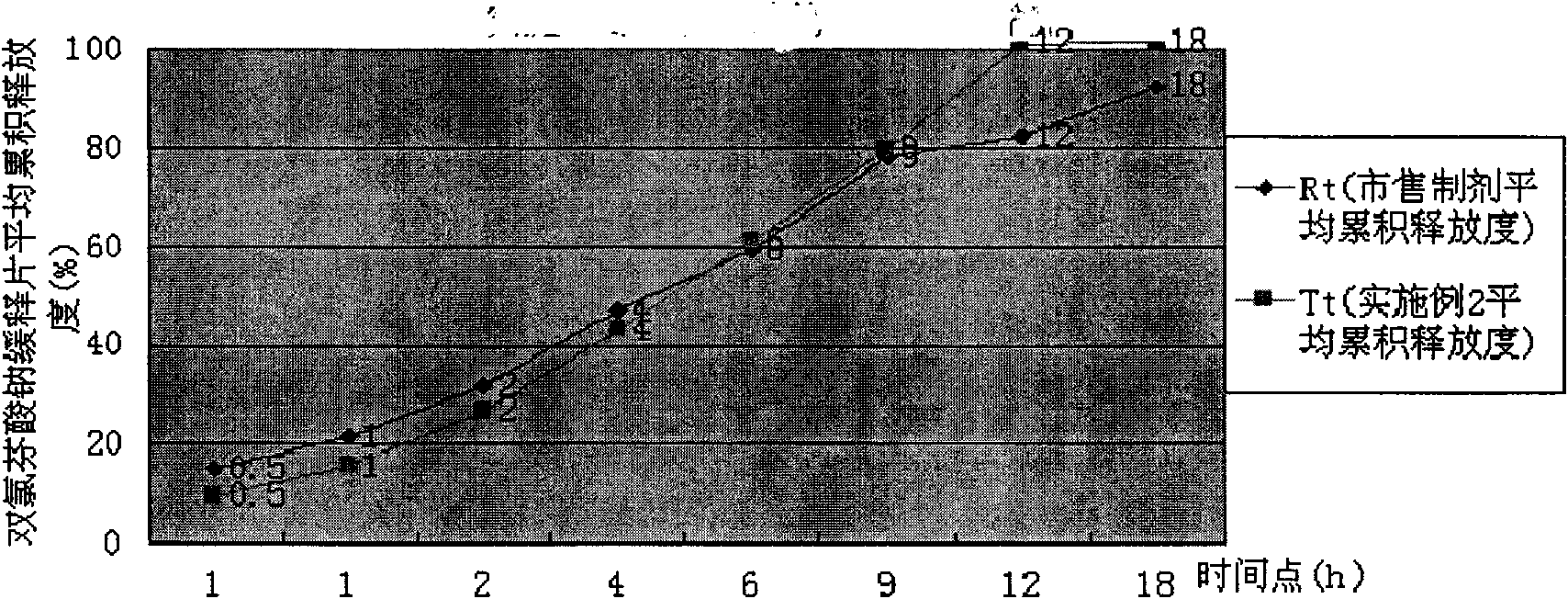
[0039] Take by weighing diclofenac sodium slow-release tablet powder 25g (wherein HPMC K15M CR accounts for 20% of powder gross weight), add 50g hot distilled water, glass rod stirs and make it disperse, add distilled water 425g, high-shear mixing emulsifier fully stirs 40 minutes, Prepare an aqueous solution containing 1% HPMC, and use a Brookfield LVDV-C digital display viscometer to select the #2 rotor at a speed of 10 rpm to measure the viscosity to be 846 centipoise. The release rate of diclofenac sodium sustained-release tablets prepared at this time is shown in Table 2, and compared with the release rate of commercially available preparations, the dissolution curve similarity f2 factor method evaluation is shown in Table 2. figure 2 .
[0040] The release degree of table 2 embodiment 2
[0041]
[0042] Conclusion: Example 2 is evaluated according to the dissolution curve similarity f2 factor, f2=54>50, it is judged that its release is similar to the commerc...
Embodiment 3
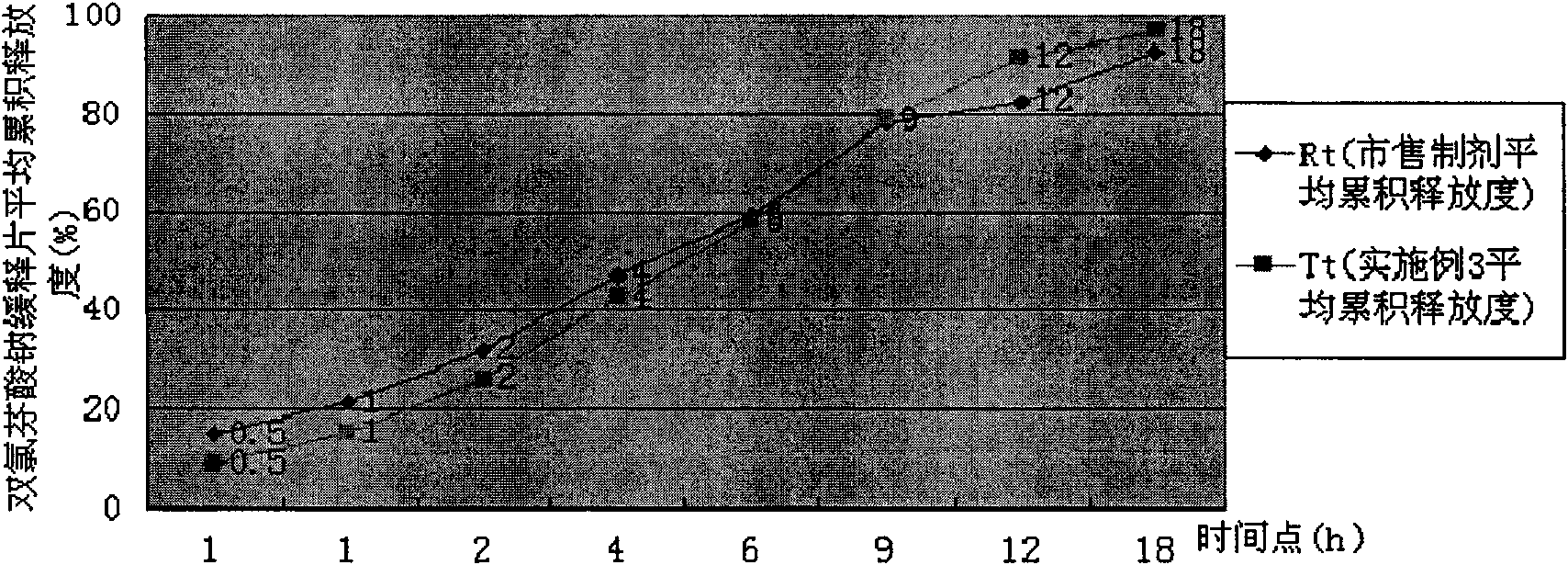
[0044] Take by weighing 20g of diclofenac sodium sustained-release tablet granules (wherein the total amount of HPMC K4M CR and HPMC K15M CR accounts for 25% of the total weight of the granules; HPMC K4M CR: HPMC K15M CR=1: 1.5), add 50g of hot distilled water, and stir with a glass rod Make it dispersed, add distilled water 430g, high-shear mixing emulsifier fully stir for 40 minutes, prepare an aqueous solution containing 1% HPMC, use Brookfield LVDV-C digital display viscometer, select #2 rotor, and measure the viscosity under the condition of rotating speed 10rpm to be 898 centimeters moor. The release rate of diclofenac sodium sustained-release tablets prepared at this time is shown in Table 3, and compared with the release rate of commercially available preparations, the dissolution curve similarity f2 factor method evaluation is shown in Table 3. image 3 .
[0045] The release degree of table 3 embodiment 3
[0046]
[0047] Conclusion: Example 3 is evaluated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com