Application of neuraminidase and neuraminidase inhibitors to preparation of medicines for inhibiting hepatic gluconeogenesis
A neuraminidase and inhibitor technology, applied in the field of biomedicine, can solve the problem of undiscovered neuraminidase correlation and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
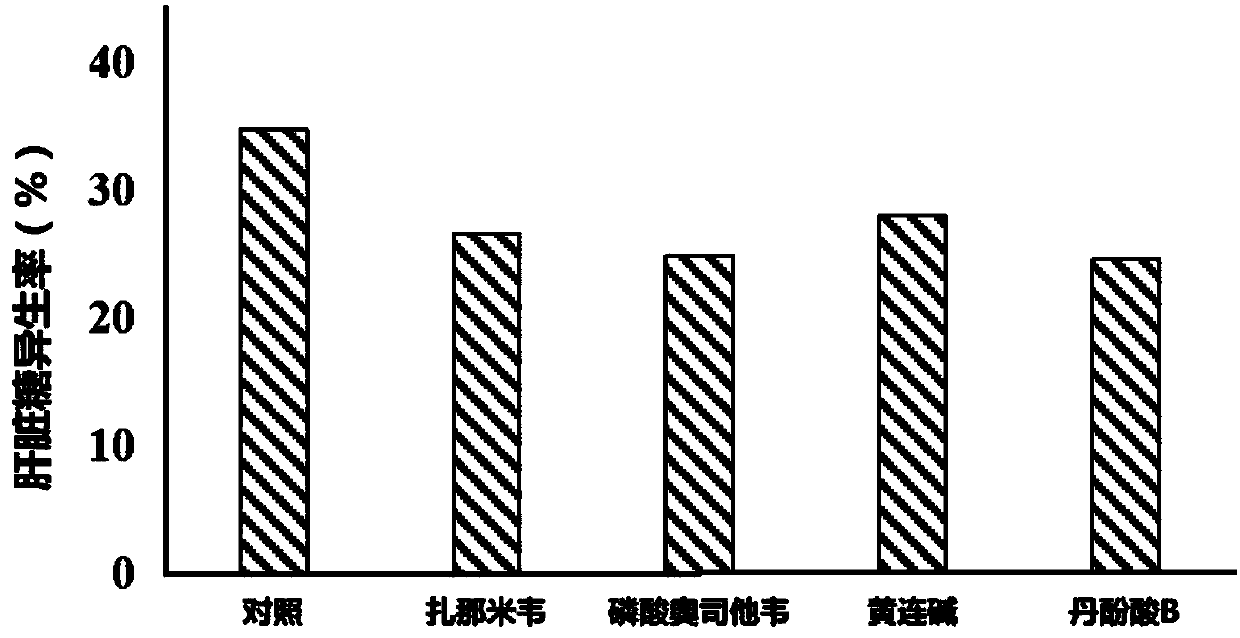
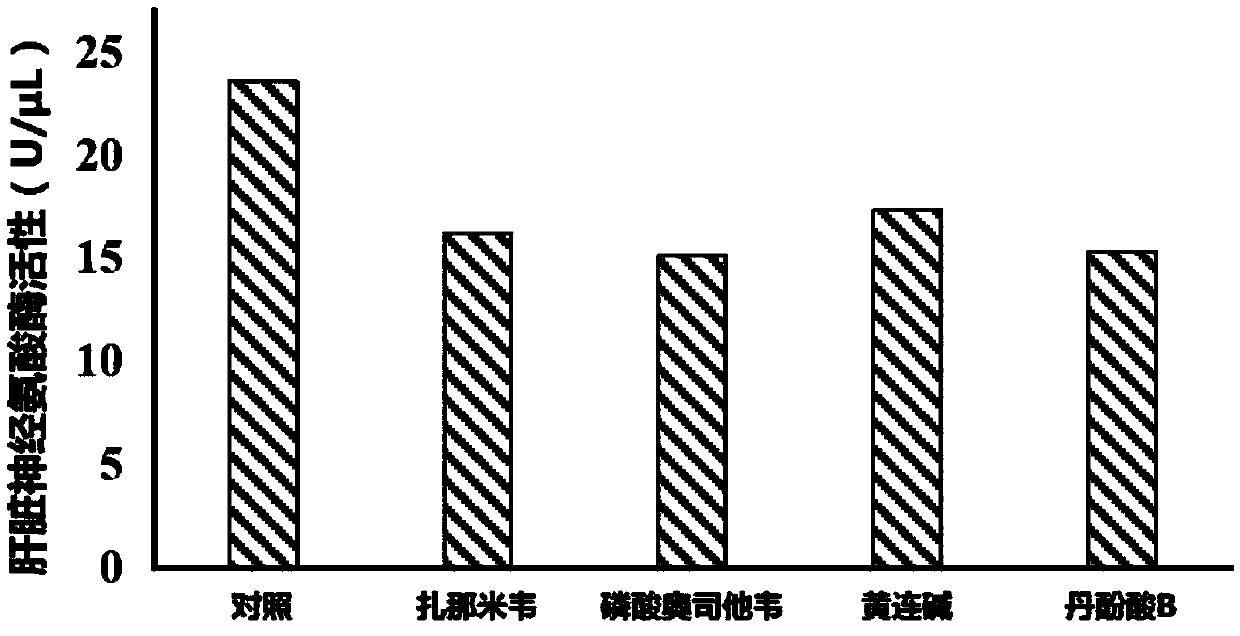
[0024] Fifty Kunming mice were randomly divided into 5 groups according to body weight and gender: control group and zanamivir, oseltamivir phosphate, coptisine, salvianolic acid B administration group. The mice in the administration group were given 0.2mg / kg / d zanamivir (i.v.), 5mg / kg / d oseltamivir phosphate (p.o.), 40mg / kg / d coptisine (p.o.), 40mg / kg / d salvianolic acid B (p.o.), the control group was given an equal volume of vehicle 0.5% CMC-Na by intragastric administration for 4 weeks. Take 7 rats from each group to measure the rate of gluconeogenesis. The specific method is: after fasting for 12 hours after the last gavage, take blood from the tail vein to measure the fasting blood sugar, which is recorded as the blood sugar value at 0 min; After injecting L-a-alanine, the eyeballs were removed 1 hour later to collect blood, and the blood glucose level was measured at 60 minutes. The gluconeogenesis rate (%) was calculated according to the blood glucose values at 0 mi...
Embodiment 2
[0032] The commercially available neuraminidase inhibitor screening kit P0309 (Beyotime, Beyotime) was used to test the inhibitory activity of salvianolic acid B in vitro, and the positive control drug was oseltamivir phosphate. Add 70 μL of buffer solution and 10 μL of neuraminidase solution to each well of a 96-well plate, then add 10 μL of different concentrations of the test solution, shake and mix, incubate at 37°C for 5 minutes, add 10 μL of the solution containing the fluorescent substrate, shake and mix Homogenize, incubate at 37°C for 30min, and perform fluorescence measurement, wherein the excitation wavelength is 322nm and the emission wavelength is 450nm. The inhibition rates of different test solutions were calculated according to the fluorescence readings, and the IC50 values of the positive control drugs oseltamivir phosphate and salvianolic acid B were further obtained. The results are shown in the table below.
[0033]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com