HPLC determination method for detecting impurities in zanamivir and zanamivir-containing preparation
A zanamivir and preparation technology, applied in the field of drug impurity analysis and detection, can solve the problems of unsuitable quantitative and qualitative use, unstable chromatographic separation, long analysis time, etc., achieve excellent retention capacity, appropriate analysis time, and stable analysis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
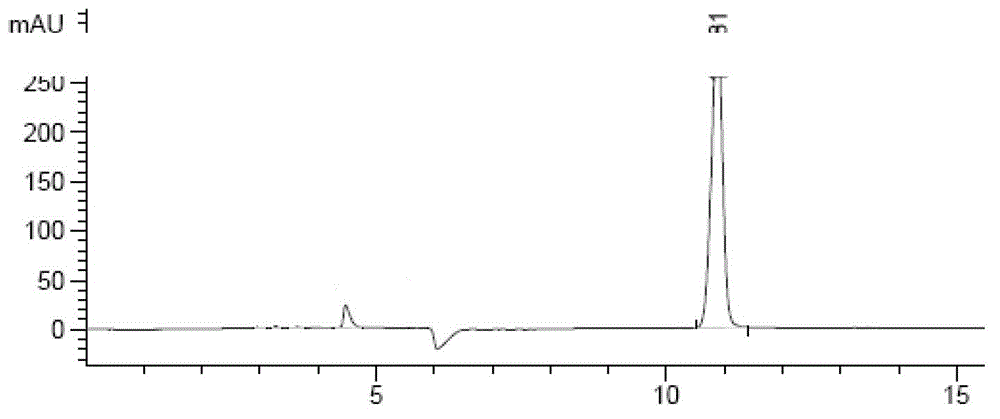
[0037] Embodiment 1: 1.1 Preparation of sample solution: take an appropriate amount of inhaled powder mist, accurately weighed, add an appropriate amount of water for ultrasonic dissolution, add mobile phase dilution to make a solution containing 20 mg of zanamivir in every 1 ml, as the test sample solution.
[0038] 1.2 Instrument and chromatographic conditions:
[0039] Instrument: Agilent 1200 high performance liquid chromatography;
[0040] Chromatographic column: Waters HILIC Silica (4.6*150mm, 3μm);
[0041] Mobile phase: acetonitrile: 10mM ammonium formate (pH6.5) = 65:35;
[0042] Flow rate: 0.5ml / min;
[0043] Column temperature: 25°C;
[0044] Detection wavelength: 210nm and 234nm;
[0045] 1.3 Determination: Take 5 μl and inject it into the high-performance liquid chromatograph, and record the spectrum (attached figure 1 , figure 2 ).
Embodiment 2
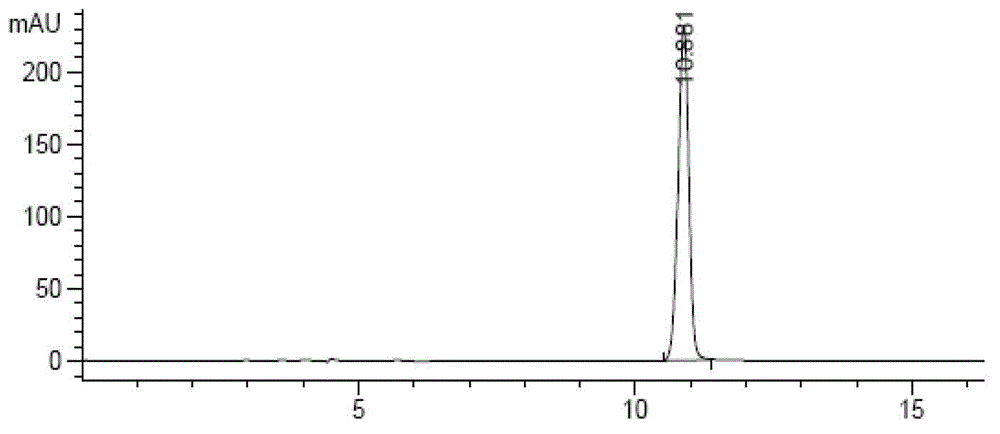
[0047] 2.1 Preparation of sample solution: Take about 10 mg of inhalation powder, add mobile phase to prepare 0.5 mg / ml test solution, add 2 ml of 0.1 mol / L hydrochloric acid solution, let it stand, add 0.1 mol / L sodium hydroxide solution in 2 ml and, as an acid-destroyed sample solution.
[0048] 2.2 Instrument and chromatographic conditions:
[0049] Instrument: Agilent 1200 high performance liquid chromatography;
[0050] Chromatographic column: Waters HILIC Silica (4.6*150mm, 3μm);
[0051] Mobile phase: acetonitrile: 10mM ammonium formate (pH6.5) = 65:35;
[0052] Flow rate: 0.5ml / min;
[0053] Column temperature: 25°C;
[0054] Detection wavelength: 210nm and 234nm;
[0055] 2.3 Determination: Take 5 μl and inject it into the high-performance liquid chromatograph, and record the spectrum (attached image 3 , Figure 4 ).
Embodiment 3
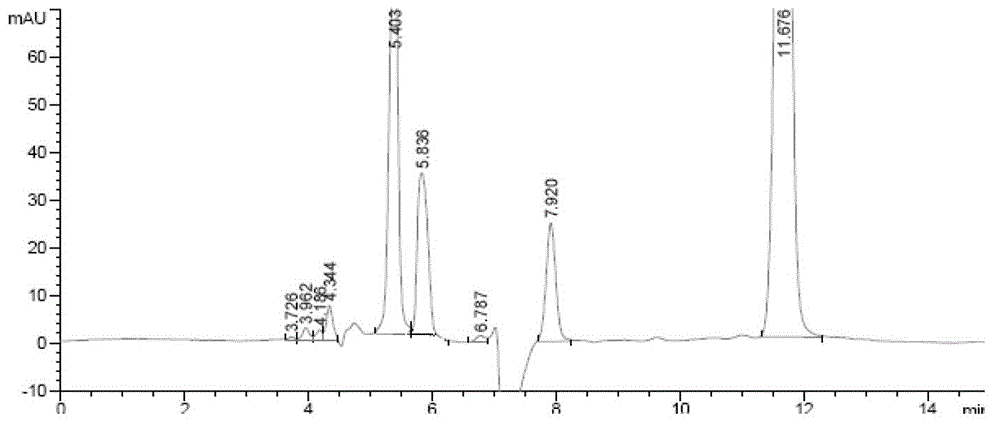
[0057] 3.1 Preparation of sample solution: Take about 10 mg of inhalation powder, add mobile phase to prepare 0.5 mg / ml test solution, add 2 ml of 0.1 mol / L sodium hydroxide solution, let it stand, add 0.1 mol / L hydrochloric acid solution in 2 ml and, as a solution of alkali-destroyed samples.
[0058] 3.2 Instrument and chromatographic conditions:
[0059] Instrument: Agilent 1200 high performance liquid chromatography;
[0060] Chromatographic column: Waters HILIC Silica (4.6*150mm, 3μm);
[0061] Mobile phase: acetonitrile: 10mM ammonium formate (pH6.5) = 65:35;
[0062] Flow rate: 0.5ml / min;
[0063] Column temperature: 25°C;
[0064] Detection wavelength: 210nm and 234nm;
[0065] 3.3 Determination: Take 5 μl and inject it into the high-performance liquid chromatograph, and record the spectrum (attached Figure 5 , Figure 6 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com