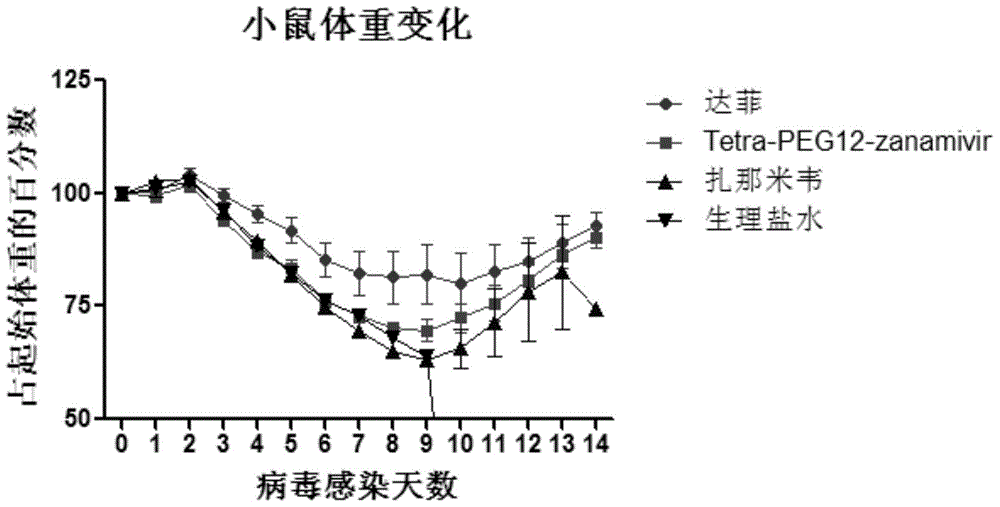
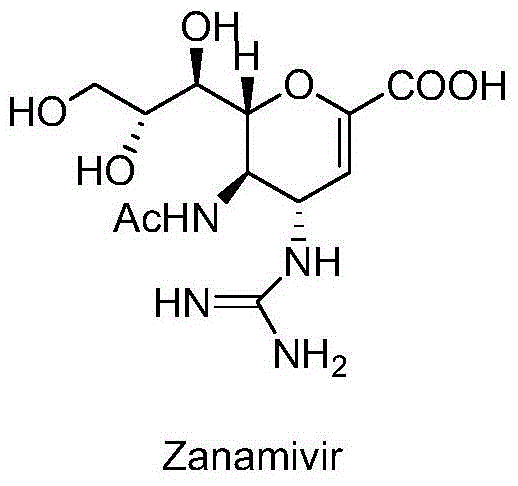
Tetravalent zanamivir and its preparation method and application
A zanamivir and reaction technology, applied in the field of medicine, can solve the problems of complicated administration routes, restricted promotion, etc., and achieve the effects of high-efficiency anti-influenza virus activity and high-efficiency inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, preparation tetravalent zanamivir
[0052] (1) Dissolve 2.8 g of triethylene glycol (m=3) in tetrahydrofuran, add 1.4 mL of triethylamine and stir for 30 min, then add 1.9 g of solid p-toluenesulfonyl chloride, react at room temperature for 16 h, filter and spin dry. Using 100-200 mesh silica gel column chromatography and gradient elution, 2.52 g of mono-p-tosylated polyethylene glycol was obtained with a yield of 58%.
[0053] The reaction equation of this step is as follows:
[0054]
[0055] (2) Dissolve 2.52 g of the mono-p-toluenesulfonated polyethylene glycol prepared in the previous step in acetonitrile, add 0.57 g of solid sodium azide, react at 80°C for 3 hours, filter and spin dry, then add a small amount of ethyl acetate ester, filtered and spin-dried to obtain 1.53 g of monoazidated polyethylene glycol, a colorless transparent liquid, with a yield of 86.5%.
[0056] The reaction equation of this step is as follows:
[0057]
[0058] (3...
Embodiment 2
[0083] Embodiment 2, the enzyme activity inhibition test of tetravalent zanamivir to NA neuraminidase
[0084] Tetra-PEG3-zanamivir, tetra-PEG6-zanamivir, tetra-PEG12-zanamivir and zanamivir (zanamivir) were prepared into 50μM, 5μM, 500μM, 50nM, 5nM, 0.5nM, 0.1nM PBS solutions. Add 10 μL of the inhibitor solution configured above and 10 μL of NA neuraminidase Tris PH=8.0 buffer to a 96well standard opaque plate and incubate for 30 min in a 37°C incubator, then add 30 μL of 167 μM 4-MUNANA fluorescent substrate Immediately put the 96-well plate into a microplate reader to measure the fluorescence value of 355-460nM for 30min. The obtained data were processed with GraphPad Prism software, and IC50 was calculated, as shown in the data in Table 1.
[0085] Table 1 Quadrivalent zanamivir molecule and zanamivir inhibitory effect on NA neuraminidase
[0086]
[0087] As can be seen from the above table, the three tetravalent zanamivir molecules tetra-PEG3-zanamivir, tetra-PEG6-z...
Embodiment 3
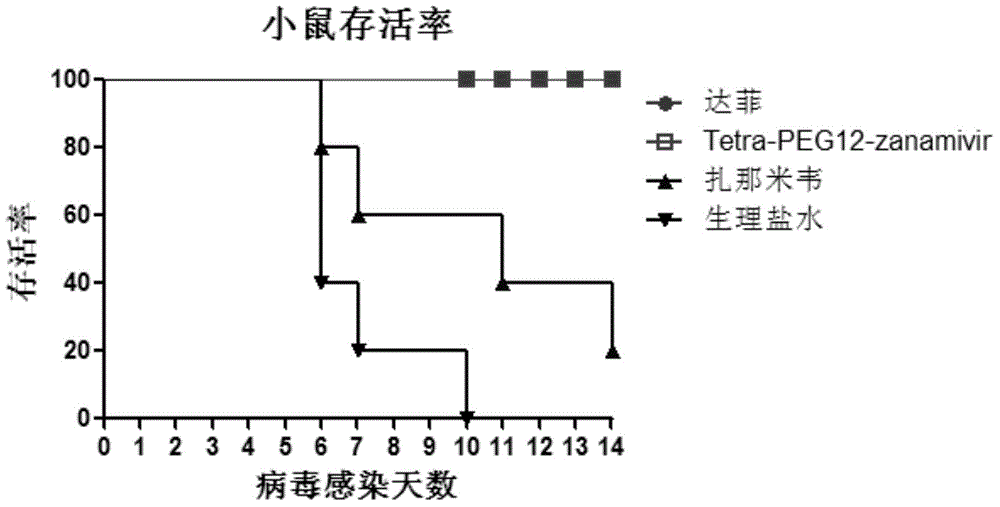
[0088] Example 3, Inhibition Test of Tetravalent Zanamivir on Influenza Virus Infected MDCK Cells
[0089] Tetra-PEG3-zanamivir, tetra-PEG6-zanamivir, tetra-PEG12-zanamivir and zanamivir were prepared in 500nM, 250nM, 125nM, 62.5nM, 31.25nM, 2nM, 1nM, 0.5nM, 0.125nM and 0.0625nM PBS solutions. MDCK cells (Beijing Jiaxin Risheng Technology Co., Ltd.) were cultured according to conventional cell culture methods. Digest MDCK cells with trypsin, divide evenly into 96-well plates, 100 μL / well, and ensure that the number of cells is (2-8)×10 5 / hole; CO 2 The cells were cultured in an incubator for 24 hours. Inactivate DMEM solutions of different concentrations in a water bath at 56°C for 30 minutes, dilute them 2-fold, mix them with an equal amount of influenza virus (diluted in serum-free DMEM medium) containing 100 TCID50, and incubate at 37°C for 1 hour. Discard the cell culture medium in the 96-well plate, add 100 μL / well of serum-virus mixture, and place in CO 2 In the ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com