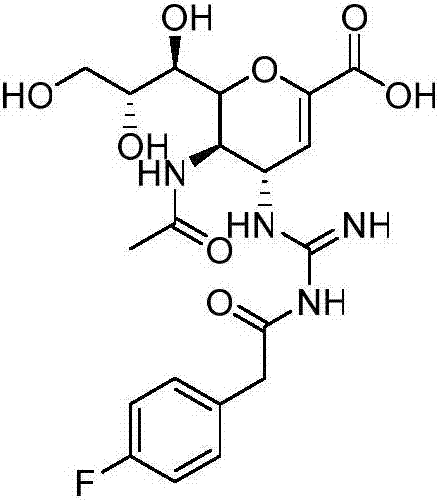
Neuraminidase inhibitor zanamivir derivative and preparation method thereof
A technology of neuraminidase and zanamivir, which is applied in the field of medicine and biology, can solve the problems of limited range of vaccine use and inability to effectively prevent and treat influenza viruses, and achieve the effect of saving time and simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
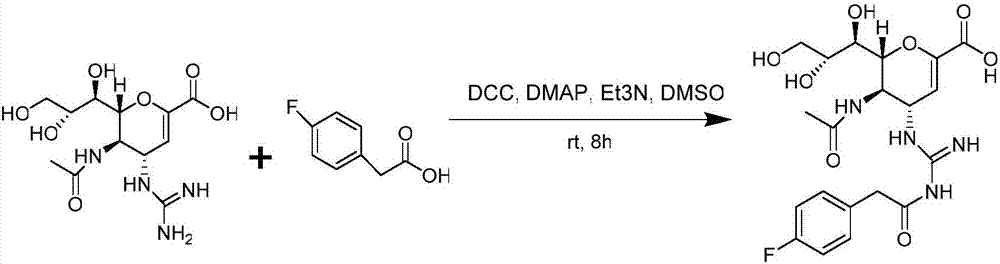
[0020] (1) Accurately weigh 118.2mg (0.767mmol) of 4-fluorophenylacetic acid and 186.1mg (0.902mmol) of DCC into a round bottom flask, then add 3.0mL of anhydrous dimethyl sulfoxide to dissolve and mix, stir evenly, and vacuumize , under nitrogen protection, and react at room temperature for 1 hour to obtain the active ester.
[0021] (2) Accurately weigh 150.0mg (0.451mmol) of zanamivir, dissolve it with 2ml dimethyl sulfoxide, slowly add it dropwise to the active ester reaction solution, then add dropwise 0.13mL triethylamine and 2.75mg (0.022mmol) DMAP, mixed, stirred evenly, vacuumed, under nitrogen protection, reacted at room temperature for 15 hours, TLC (ethyl hexanoate:methanol:water=4:2:1) monitored the completion of the reaction.
[0022] (3) After the reaction is over, filter with medium-speed filter paper to remove most of the urea by-products; add dichloromethane and water to the filtrate, extract, remove unreacted 4-fluorophenylacetic acid and active esters, and ...
Embodiment 2
[0025] (1) Accurately weigh 118.2mg (0.767mmol) of 4-fluorophenylacetic acid, 172.9mg (0.902mmol) of EDC, and 121.9mg (0.902mmol) of HoBt into a round bottom flask, then add 3.0mL of anhydrous dimethyl sulfoxide Dissolve and mix, stir evenly, vacuumize, protect with nitrogen, and react at room temperature for 1 hour to obtain the active ester.
[0026] (2) Accurately weigh 150.0 mg (0.451 mmol) of zanamivir, dissolve it with 2 mL of dimethyl sulfoxide, slowly add it dropwise to the active ester reaction solution, and then add dropwise 0.13 mL of triethylamine and 2.75 mg (0.022 mmol) DMAP, mixed, stirred evenly, vacuumed, under nitrogen protection, reacted at room temperature for 15 hours, TLC (ethyl hexanoate:methanol:water=4:2:1) monitored the completion of the reaction.
[0027] (3) After the reaction, add dichloromethane and water to the reaction solution, extract, remove unreacted 4-fluorophenylacetic acid and active ester, obtain the water phase, and remove the dichlorom...
Embodiment 3
[0029] Same as in Example 1, in the first step reaction to generate the active ester reaction solution, under nitrogen protection, react at room temperature for half an hour to obtain the active ester, and the final product yield is 61.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com