Application of zanamivir in preparation of medicine for treating or preventing preeclampsia
A technology for zanamivir and preeclampsia is applied in the field of use of zanamivir in the preparation of drugs for treating or preventing preeclampsia, and can solve the problems of lack of treatment means, unclear pathogenesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Establishment of an animal model of preeclampsia
[0058] Pregnant CD-1 mice (aged 8-10 weeks) were randomly divided into control group and preeclampsia model group. On days 7-16 of the gestational cycle, preeclampsia was induced in mice in the model group by subcutaneous injection of 50 mg / kg of N-nitro-L-arginine methyl ester (L-NAME) every day. In the control group, an equal volume of solvent was injected subcutaneously.
Embodiment 2
[0059] Example 2 Experimental grouping
[0060]
[0061]
Embodiment 3
[0062] Example 3 Evaluation of therapeutic effect
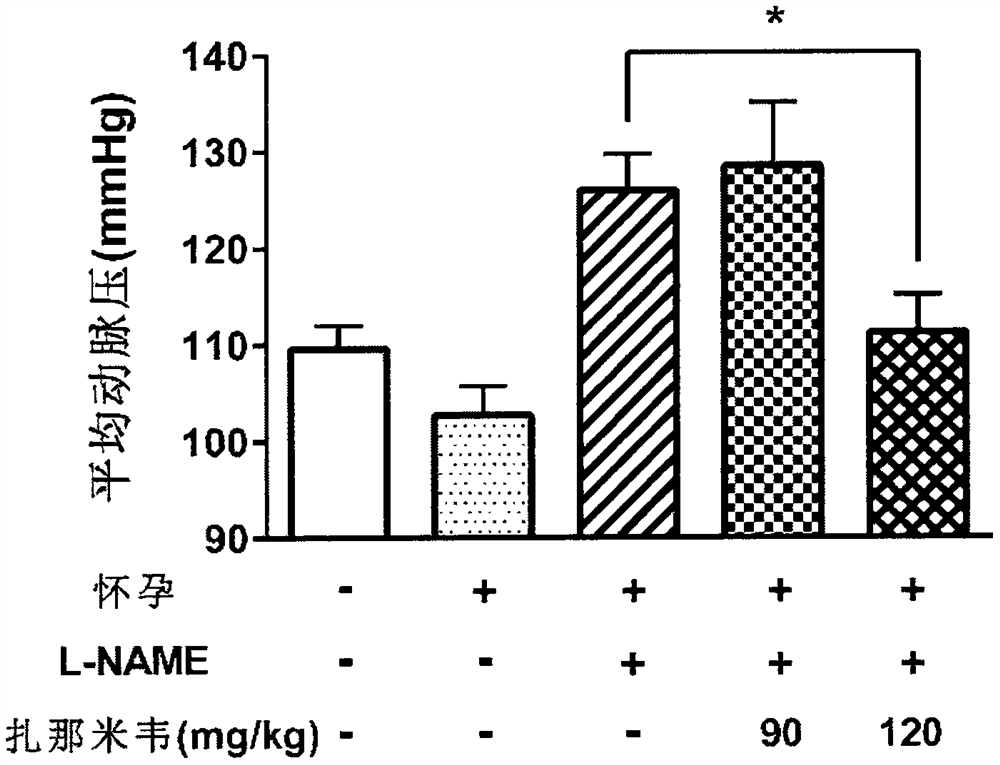
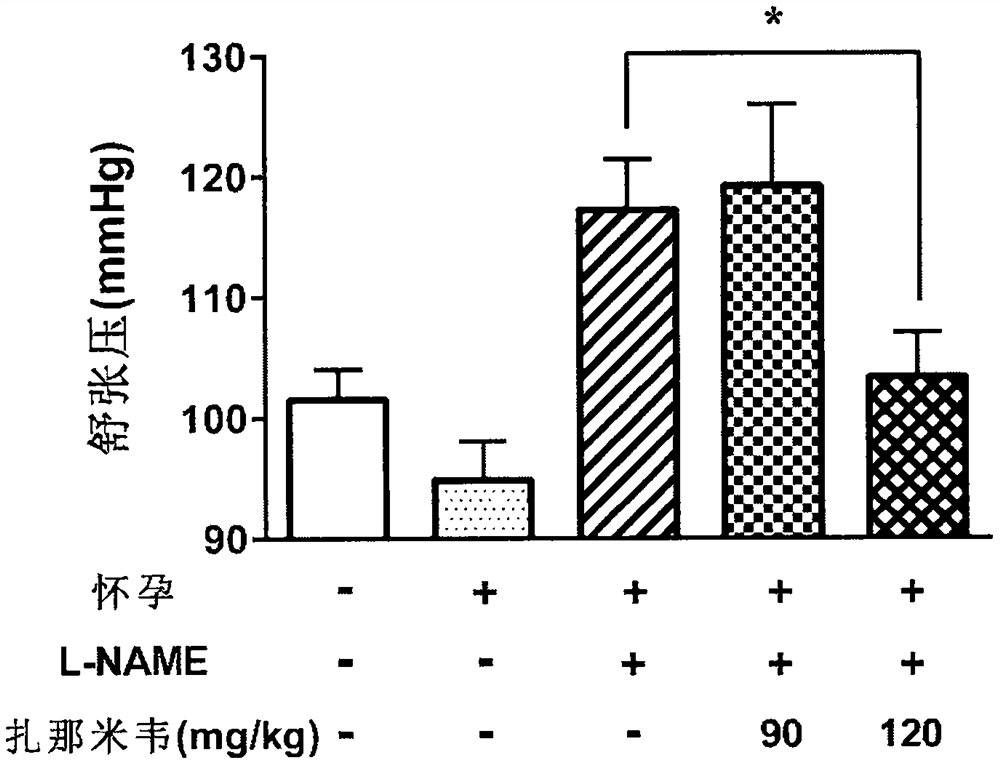
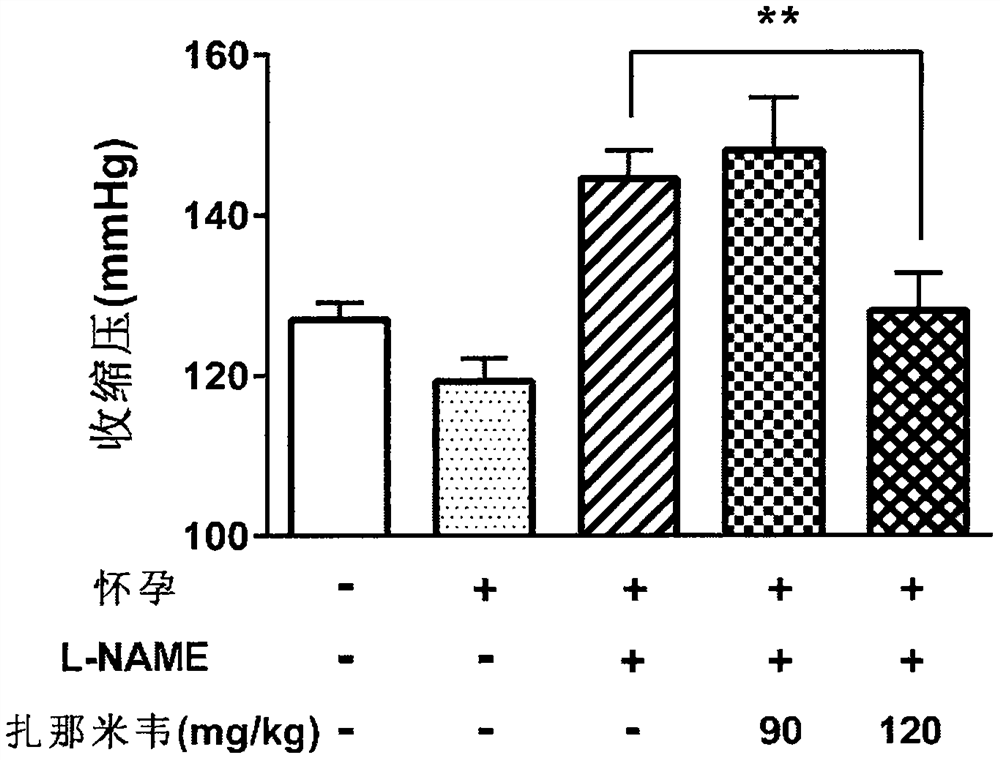
[0063] In L-NAME-induced preeclampsia animal models, according to literature reports, symptoms of hypertension appear two days after the first administration of L-NAME, and persist until at least five days after the first administration of L-NAME. Symptoms of proteinuria appear relatively late. Therefore, in this experiment, we focused on monitoring blood pressure changes in pregnant mice on the 12th day of pregnancy (that is, five days after the first administration of L-NAME), as well as collecting 24-hour urine on the 17th-18th day of pregnancy and measuring the level of total protein . At the same time, we also recorded the number of pups produced by each group of pregnant mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com