Anti-influenza virus compound as well as preparation method and application thereof
An anti-influenza virus and compound technology, applied in antiviral agents, steroids, organic chemistry, etc., can solve problems such as restrictions on promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
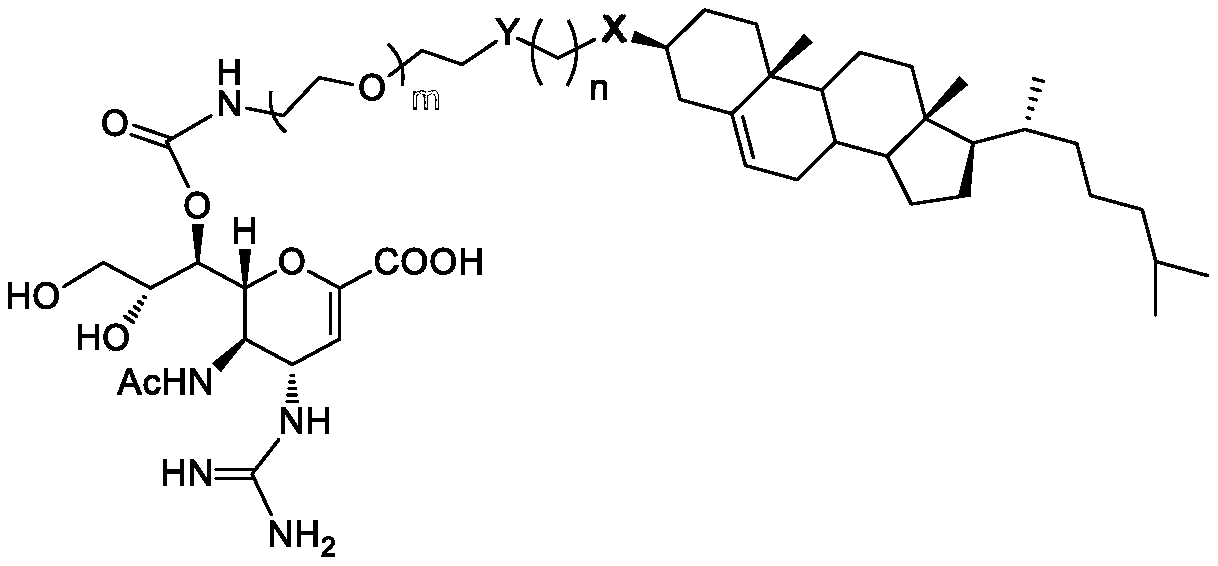
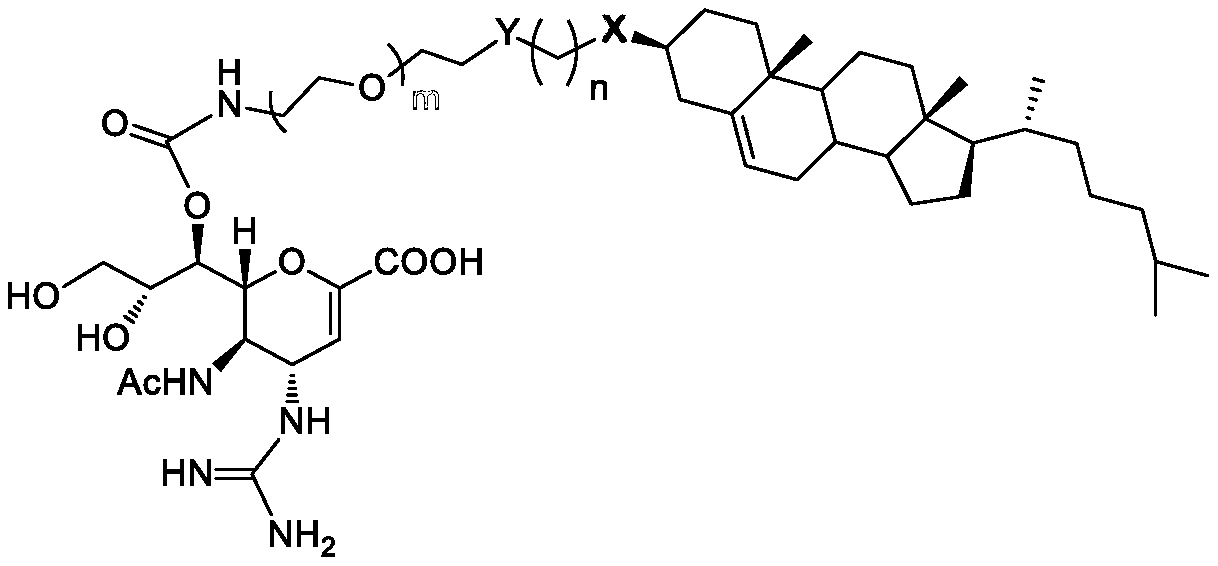
[0079] Experimental example 1, the specific synthetic operation of the anti-influenza virus compound of the present invention
[0080]
[0081] When R of compound I is Ts, the reaction conditions a include: compound I (R=Ts, 1mol) and compound II (1.2mol) are dissolved in 1,4-dioxane (100mL), 125°C Under reflux reaction overnight. After the solution is concentrated (when Y 1 =SAc, the concentrate needs to be treated with 1N sodium methoxide / methanol solution to remove the acetyl group on SAc) and separated by silica gel column chromatography to obtain compound III as a white solid with a yield of 78-86%.
[0082] When R of compound I is ClCO, the reaction condition a includes: compound I (R=ClCO, 1 mol) and compound II (1.2 mol) are dissolved in pyridine (100 mL), and stirred at room temperature overnight. Methanol (2 mL) was added to the reaction solution, concentrated and dissolved in dichloromethane, and successively washed with 1M HCl solution and 1M NaHCO 3 The solu...
experiment example 2
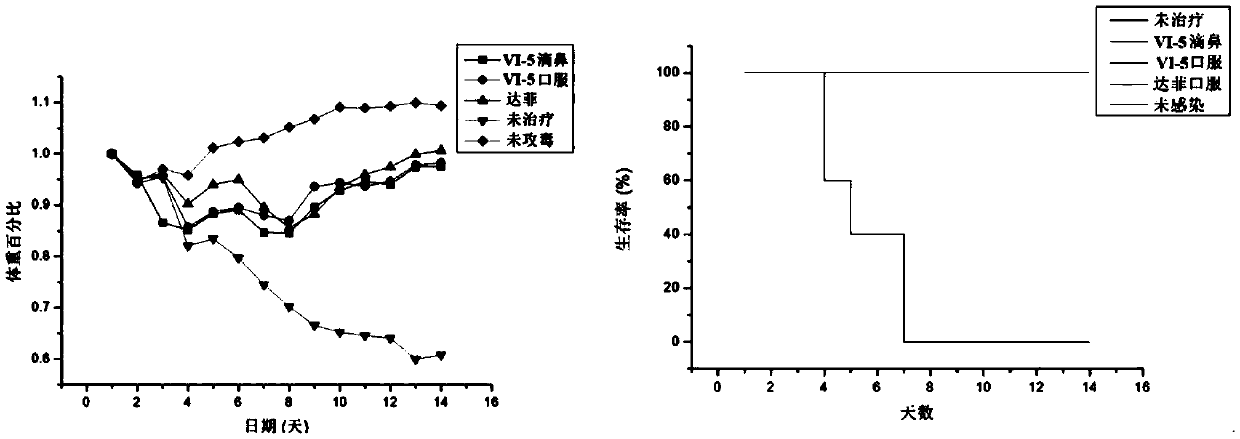
[0087] Experimental example 2, the activity evaluation of anti-influenza virus compound of the present invention:
[0088] NA enzyme activity inhibition test: the purified NA protein (N1, N5, etc.) was diluted 5×, 25×, 125×, 625×, 3125×, 15625× with 20 / 150 Tris / NaCl with a pH value of 8.0 Afterwards, 10 μL of NA solutions of different concentrations and 10 μL of PBS were added to the black 96-well ELISA plate, and incubated in a constant temperature incubator at 37°C for 30 min, and then 30 μL of 167 μM 4-MUNANA (4-methylumbelliferyl-N -acetylneuraminic acid) fluorescent substrate. The fluorescence value per minute (excitation wavelengths 355nm, emission wavelengths 460nm) was measured with a microplate reader for a total of 30 minutes. From the results, the concentration of NA whose fluorescence value increased linearly within 30 minutes and did not exceed 5000RU was selected as the concentration of the enzyme activity inhibition experiment. Inhibitor (VI) was diluted with ...
experiment example 3
[0105] Experimental example 3, the determination of the lipid-water partition coefficient of the anti-influenza virus compound of the present invention
[0106] Shake n-octanol and double-distilled water with a constant temperature (37±1)°C shaker at room temperature for 24h to make them mutually saturated. After standing overnight for stratification, the two phases were separated and stored for future use. Accurately weigh an appropriate amount of the compound to be tested in a 10 mL volumetric flask, dissolve in n-octanol saturated with water, oscillate ultrasonically for 30 min, and constant volume to obtain a mother solution with a concentration of 1 mmol / L. Precisely measure a certain amount of the total solution with a pipette gun into a 10mL volumetric flask, dilute with n-octanol saturated with water and set the volume to a series concentration of 10-100 μmol / L, the concentration increase gradient is 10 μmol / L, respectively at 200-400nm Scan within the wavelength rang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com