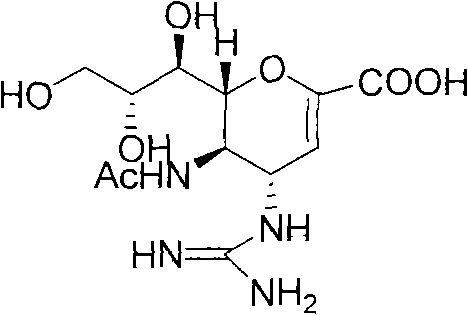
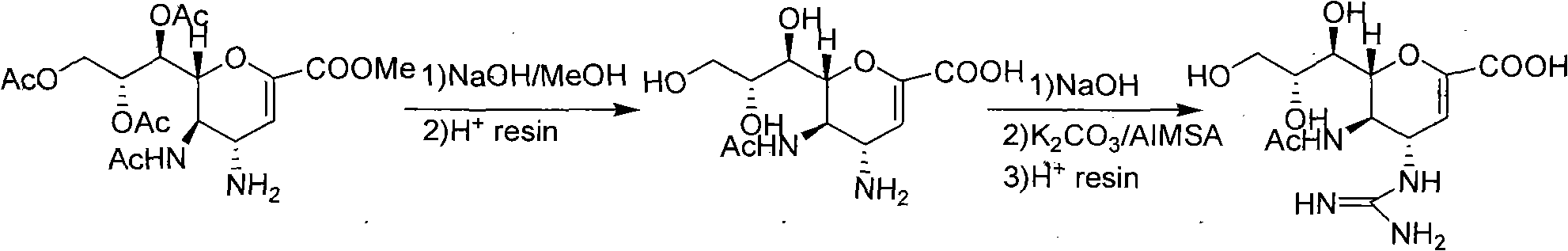
Preparation method of zanamivir
A zanamivir and compound technology, applied in the field of preparation of antiviral drug zanamivir, can solve problems affecting application, etc., and achieve the effects of simplifying operation, reducing cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
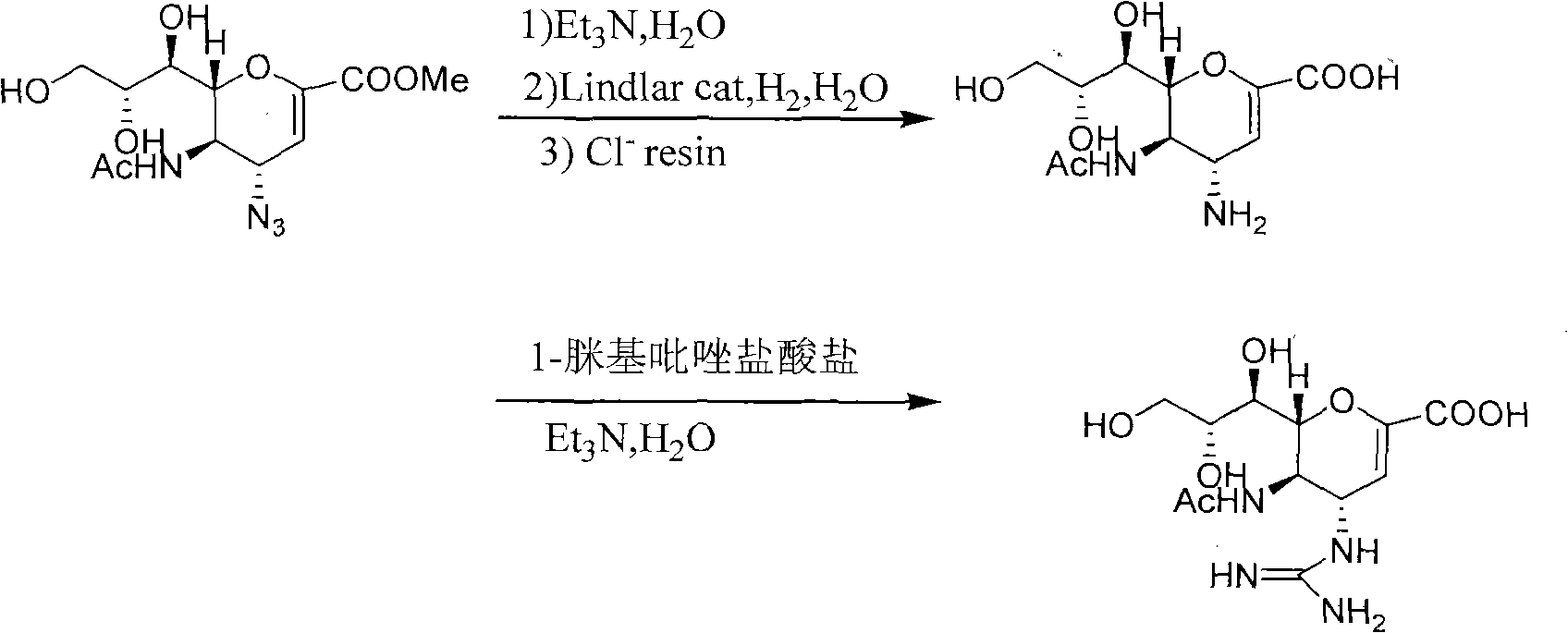
[0039] Example 1 (4S, 5R, 6R)-5-acetylamino-4-guanidino-6-((1R, 2R)-1,2,3-trihydroxy-propyl)-5,6-dihydro- Preparation of 4H-pyran-2-carboxylic acid methyl ester
[0040]
[0041] 45.6 g of (4S, 5R, 6R)-5-acetylamino-4-amino-6-((1R, 2R)-1,2,3-trihydroxy-propyl)-5,6-dihydro- Methyl 4H-pyran-2-carboxylate was dissolved in 500 mL of methanol, and then 108 g of triethylenediamine and 44 g of 1-formamidine pyrazole hydrochloride were added to the above reaction solution. Reacted at 50°C for 26h, and the resulting methanol solution was concentrated to dryness and used directly for the next reaction.
Embodiment 2
[0042] Example 2 Preparation of Zanamivir
[0043]
[0044] 300 mL of water was added to the solid prepared in Example 1, stirred to dissolve, then 120 mL of triethylamine was added, and the reaction was carried out at room temperature for 6 h. After the reaction was completed, it was concentrated to dryness. Then add water to dissolve at 50°C, add 900mL of methanol to cool down and slowly precipitate solid. Stir overnight, filter to obtain 36g zanamivir crude product, recrystallize 2 times with isopropanol-water (1: 1), dry under reduced pressure at 30 ℃, obtain white solid zanamivir (27.9g, total yield 56%) , purity>96%).
Embodiment 3
[0045] Example 3 (4S, 5R, 6R)-5-acetylamino-4-guanidino-6-((1R, 2R)-1,2,3-trihydroxy-propyl)-5,6-dihydro- Preparation of 4H-pyran-2-carboxylic acid methyl ester
[0046]
[0047] 4.56 g of (4S, 5R, 6R)-5-acetylamino-4-amino-6-((1R, 2R)-1,2,3-trihydroxy-propyl)-5,6-dihydro- Methyl 4H-pyran-2-carboxylate was dissolved in 50 mL of methanol, and then 10.8 g of triethylenediamine and 4.4 g of 1-formamidine pyrazole hydrochloride were added to the above reaction solution. Reaction at 50°C for 26h. The resulting methanol solution was concentrated to dryness, and subjected to column chromatography with 200-300 mesh silica gel, gradient elution, eluting with methanol:dichloromethane=1:5-1:3 to obtain 4.67 g of the title compound , yield 90%.
[0048] ESIMS m / z calcd for C 13 h 22 N 4 o 7 ;[M+H] + :347.15, found: 347.2;
[0049] 1 H-NMR (500MHz, DMSO-d 6 ): δ7.87 (1H, d, J = 9Hz, NH), 5.71 (1H, d, J = 2.5Hz, 3-H), 4.71 (1H, d, J = 4Hz, OH), 4.67-4.65 ( 2H, m, OH, 4-H), 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com